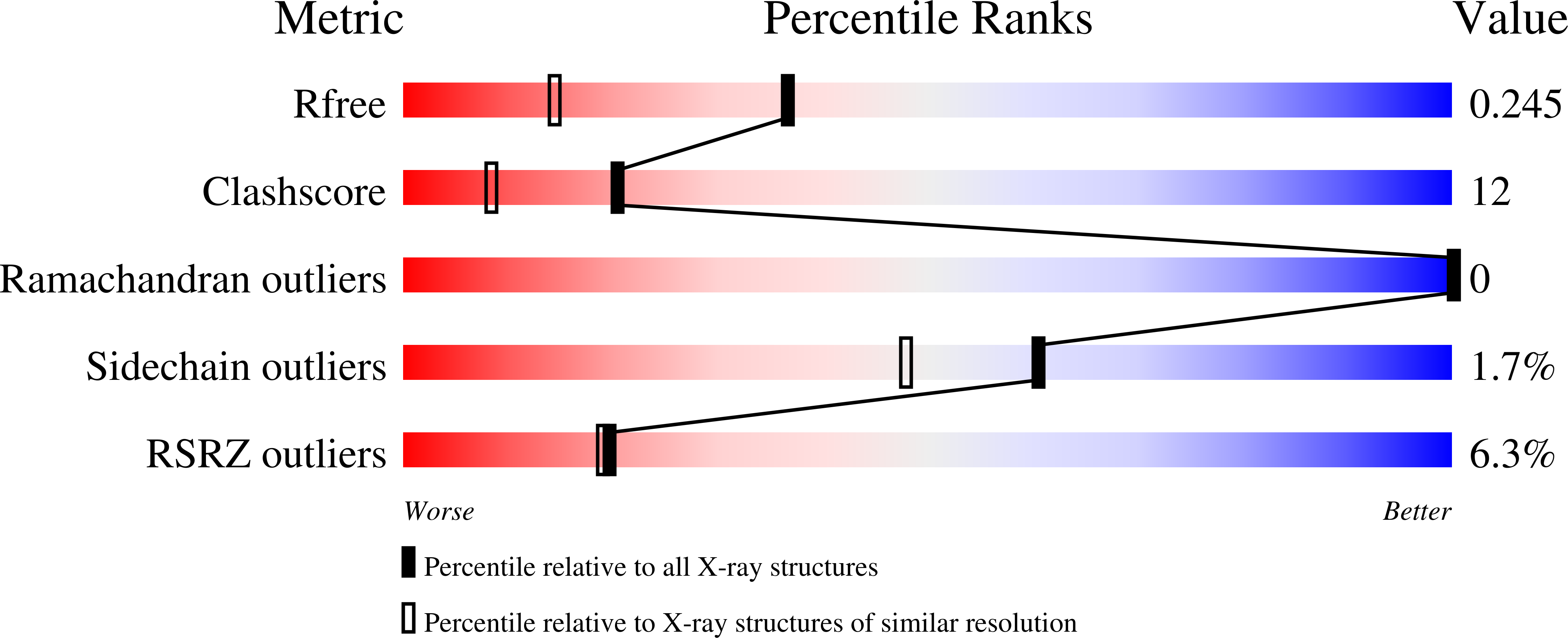
The FapF Amyloid Secretion Transporter Possesses an Atypical Asymmetric Coiled Coil.
Rouse, S.L., Stylianou, F., Wu, H.Y.G., Berry, J.L., Sewell, L., Morgan, R.M.L., Sauerwein, A.C., Matthews, S.(2018) J Mol Biol 430: 3863-3871
- PubMed: 29886016
- DOI: https://doi.org/10.1016/j.jmb.2018.06.007
- Primary Citation of Related Structures:
6FUE - PubMed Abstract:
Gram-negative bacteria possess specialized biogenesis machineries that facilitate the export of amyloid subunits, the fibers of which are key components of their biofilm matrix. The secretion of bacterial functional amyloid requires a specialized outer-membrane protein channel through which unfolded amyloid substrates are translocated. We previously reported the crystal structure of the membrane-spanning domain of the amyloid subunit transporter FapF from Pseudomonas. However, the structure of the periplasmic domain, which is essential for amyloid transport, is yet to be determined. Here, we present the crystal structure of the N-terminal periplasmic domain at 1.8-Å resolution. This domain forms a novel asymmetric trimeric coiled coil that possesses a single buried tyrosine residue as well as an extensive hydrogen-bonding network within a glutamine layer. This new structural insight allows us to understand this newly described functional amyloid secretion system in greater detail.
Organizational Affiliation:
Department of Life Sciences, Imperial College London, South Kensington Campus, London SW7 2AZ, UK.