Biophysical and structural characterization of the putative nickel chaperone CooT from Carboxydothermus hydrogenoformans.
Alfano, M., Perard, J., Miras, R., Catty, P., Cavazza, C.(2018) J Biol Inorg Chem 23: 809-817
- PubMed: 29882029
- DOI: https://doi.org/10.1007/s00775-018-1576-2
- Primary Citation of Related Structures:
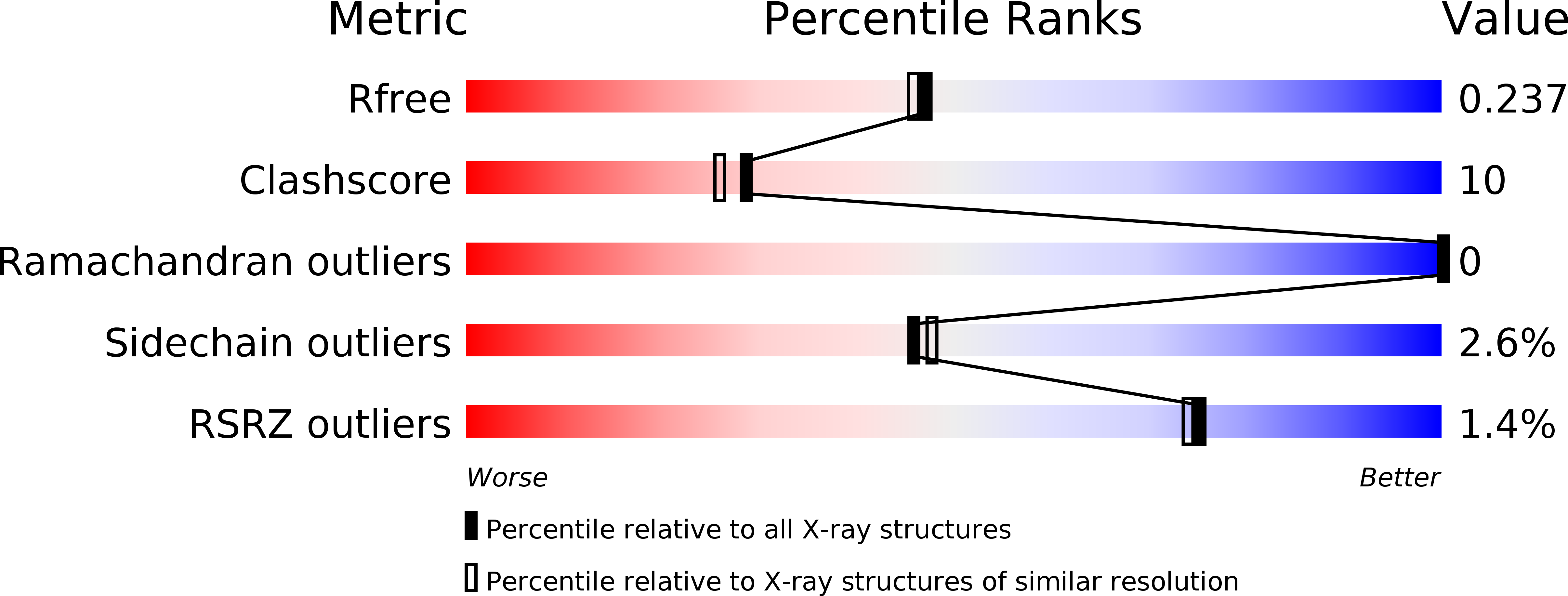
6FAN - PubMed Abstract:
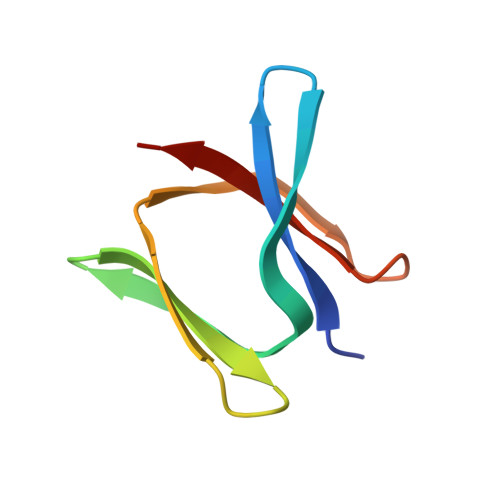
Carboxydothermus hydrogenoformans is a model microorganism for the study of [NiFe]-CODH, a key enzyme of carbon cycle in anaerobic microorganisms. The enzyme possesses a unique active site (C-cluster), constituted of a distorted [NiFe 3 S 4 ] cubane linked to a mononuclear Fe(II) center. Both the biogenesis of the C-cluster and the activation of CODH by nickel insertion remain unclear. Among the three accessory proteins thought to play a role in this latter step (CooC, CooJ, and CooT), CooT is identified as a nickel chaperone involved in CODH maturation in Rhodospirillum rubrum. Here, we structurally and biophysically characterized a putative CooT protein present in C. hydrogenoformans (pChCooT). Despite the low sequence homologies between CooT from R. rubrum (RrCooT) and pChCooT (19% sequence identity), the two proteins share several similarities, such as their overall structure and a solvent-exposed Ni(II)-binding site at the dimer interface. Moreover, the X-ray structure of pChCooT reveals the proximity between the histidine 55, a potential nickel-coordinating residue, and the cysteine 2, a highly conserved key residue in Ni(II)-binding.
Organizational Affiliation:
University of Grenoble Alpes, CEA, CNRS, BIG, CBM, 38000, Grenoble, France.