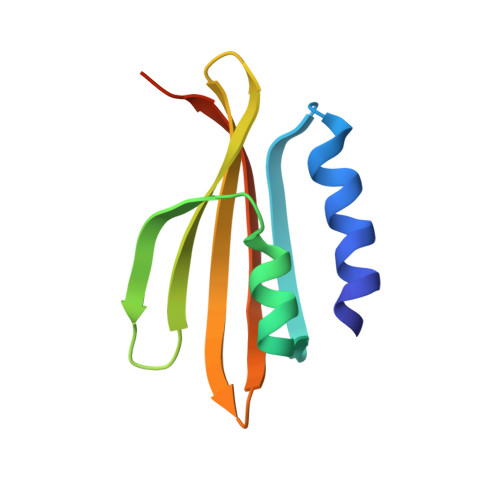
Anti-CRISPR proteins encoded by archaeal lytic viruses inhibit subtype I-D immunity.
He, F., Bhoobalan-Chitty, Y., Van, L.B., Kjeldsen, A.L., Dedola, M., Makarova, K.S., Koonin, E.V., Brodersen, D.E., Peng, X.(2018) Nat Microbiol 3: 461-469
- PubMed: 29507349
- DOI: https://doi.org/10.1038/s41564-018-0120-z
- Primary Citation of Related Structures:
6EXP - PubMed Abstract:
Viruses employ a range of strategies to counteract the prokaryotic adaptive immune system, clustered regularly interspaced short palindromic repeats and CRISPR-associated proteins (CRISPR-Cas), including mutational escape and physical blocking of enzymatic function using anti-CRISPR proteins (Acrs). Acrs have been found in many bacteriophages but so far not in archaeal viruses, despite the near ubiquity of CRISPR-Cas systems in archaea. Here, we report the functional and structural characterization of two archaeal Acrs from the lytic rudiviruses, SIRV2 and SIRV3. We show that a 4 kb deletion in the SIRV2 genome dramatically reduces infectivity in Sulfolobus islandicus LAL14/1 that carries functional CRISPR-Cas subtypes I-A, I-D and III-B. Subsequent insertion of a single gene from SIRV3, gp02 (AcrID1), which is conserved in the deleted fragment, successfully restored infectivity. We demonstrate that AcrID1 protein inhibits the CRISPR-Cas subtype I-D system by interacting directly with Cas10d protein, which is required for the interference stage. Sequence and structural analysis of AcrID1 show that it belongs to a conserved family of compact, dimeric αβ-sandwich proteins characterized by extreme pH and temperature stability and a tendency to form protein fibres. We identify about 50 homologues of AcrID1 in four archaeal viral families demonstrating the broad distribution of this group of anti-CRISPR proteins.
Organizational Affiliation:
Danish Archaea Centre, Department of Biology, University of Copenhagen, Copenhagen, Denmark.