Neuron-Subtype-Specific Expression, Interaction Affinities, and Specificity Determinants of DIP/Dpr Cell Recognition Proteins.
Cosmanescu, F., Katsamba, P.S., Sergeeva, A.P., Ahlsen, G., Patel, S.D., Brewer, J.J., Tan, L., Xu, S., Xiao, Q., Nagarkar-Jaiswal, S., Nern, A., Bellen, H.J., Zipursky, S.L., Honig, B., Shapiro, L.(2018) Neuron 100: 1385
- PubMed: 30467080
- DOI: https://doi.org/10.1016/j.neuron.2018.10.046
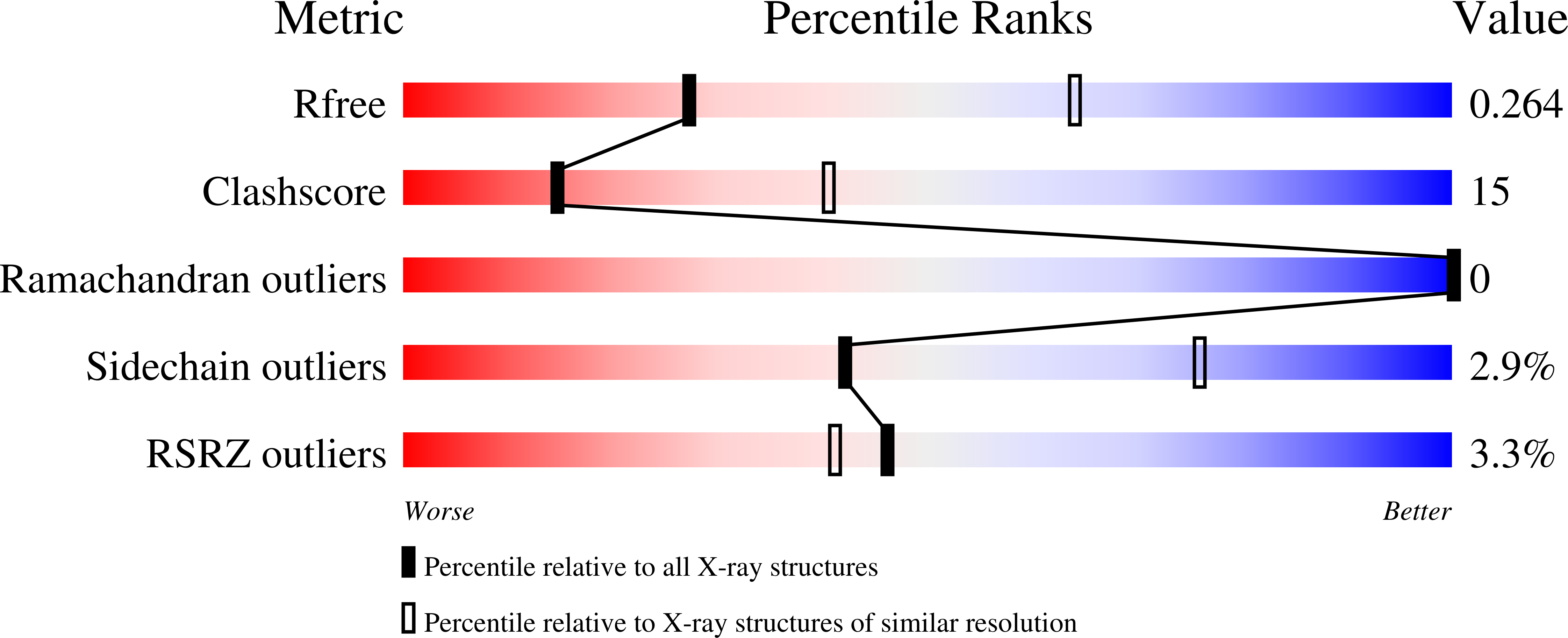
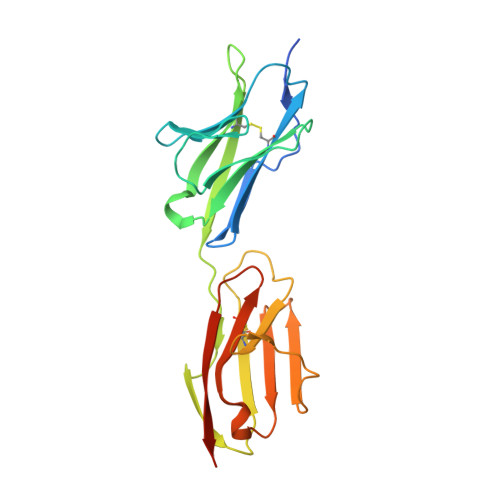
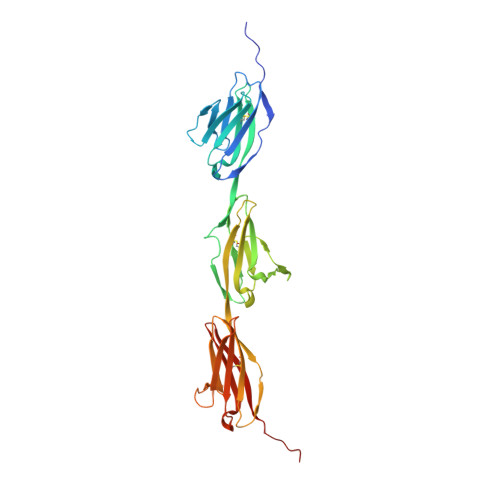
- Primary Citation of Related Structures:
6EFY, 6EFZ, 6EG0, 6EG1 - PubMed Abstract:
Binding between DIP and Dpr neuronal recognition proteins has been proposed to regulate synaptic connections between lamina and medulla neurons in the Drosophila visual system. Each lamina neuron was previously shown to express many Dprs. Here, we demonstrate, by contrast, that their synaptic partners typically express one or two DIPs, with binding specificities matched to the lamina neuron-expressed Dprs. A deeper understanding of the molecular logic of DIP/Dpr interaction requires quantitative studies on the properties of these proteins. We thus generated a quantitative affinity-based DIP/Dpr interactome for all DIP/Dpr protein family members. This revealed a broad range of affinities and identified homophilic binding for some DIPs and some Dprs. These data, along with full-length ectodomain DIP/Dpr and DIP/DIP crystal structures, led to the identification of molecular determinants of DIP/Dpr specificity. This structural knowledge, along with a comprehensive set of quantitative binding affinities, provides new tools for functional studies in vivo.
Organizational Affiliation:
Department of Biochemistry and Molecular Biophysics, Columbia University, New York, NY 10032, USA; Zuckerman Mind Brain Behavior Institute, Columbia University, New York, NY 10027, USA.