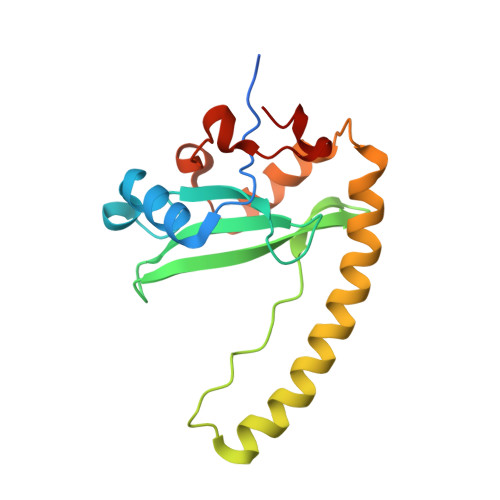
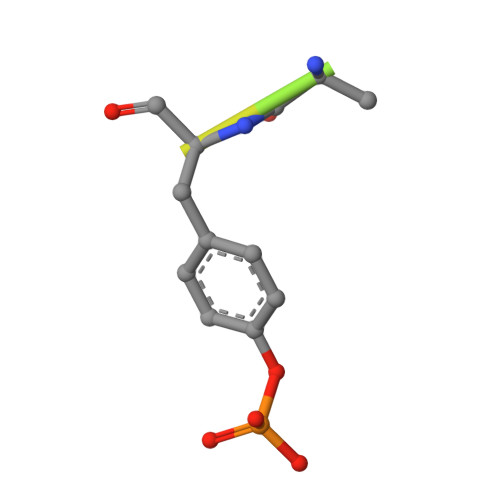
Identification and characterization of a large family of superbinding bacterial SH2 domains.
Kaneko, T., Stogios, P.J., Ruan, X., Voss, C., Evdokimova, E., Skarina, T., Chung, A., Liu, X., Li, L., Savchenko, A., Ensminger, A.W., Li, S.S.(2018) Nat Commun 9: 4549-4549
- PubMed: 30382091
- DOI: https://doi.org/10.1038/s41467-018-06943-2
- Primary Citation of Related Structures:
6E8H, 6E8I, 6E8K, 6E8M - PubMed Abstract:
Src homology 2 (SH2) domains play a critical role in signal transduction in mammalian cells by binding to phosphorylated Tyr (pTyr). Apart from a few isolated cases in viruses, no functional SH2 domain has been identified to date in prokaryotes. Here we identify 93 SH2 domains from Legionella that are distinct in sequence and specificity from mammalian SH2 domains. The bacterial SH2 domains are not only capable of binding proteins or peptides in a Tyr phosphorylation-dependent manner, some bind pTyr itself with micromolar affinities, a property not observed for mammalian SH2 domains. The Legionella SH2 domains feature the SH2 fold and a pTyr-binding pocket, but lack a specificity pocket found in a typical mammalian SH2 domain for recognition of sequences flanking the pTyr residue. Our work expands the boundary of phosphotyrosine signalling to prokaryotes, suggesting that some bacterial effector proteins have acquired pTyr-superbinding characteristics to facilitate bacterium-host interactions.
Organizational Affiliation:
Department of Biochemistry, Schulich School of Medicine and Dentistry, Western University, London, ON, N6A 5C1, Canada.