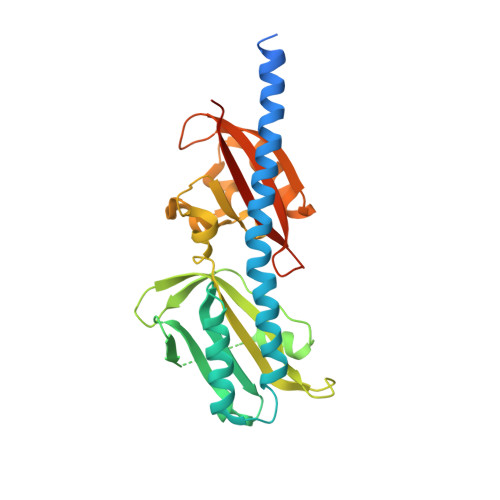
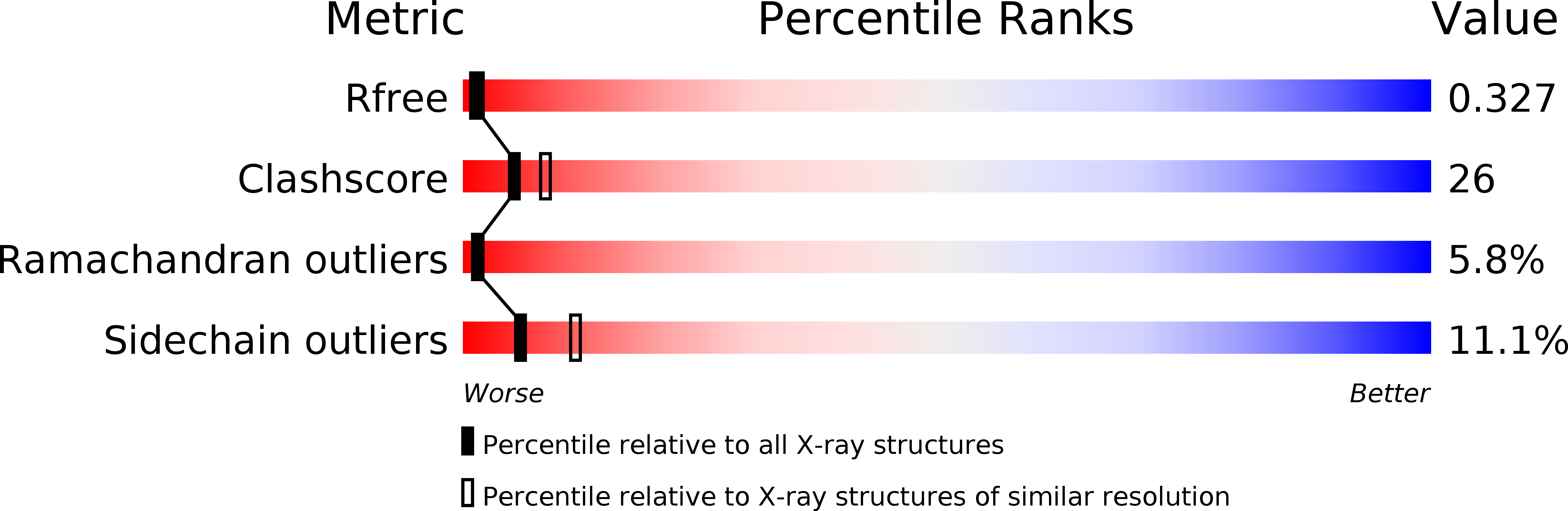Structures of the ligand-binding domain of Helicobacter pylori chemoreceptor TlpA.
Sweeney, E.G., Perkins, A., Kallio, K., James Remington, S., Guillemin, K.(2018) Protein Sci 27: 1961-1968
- PubMed: 30171638
- DOI: https://doi.org/10.1002/pro.3503
- Primary Citation of Related Structures:
6DTM, 6E09, 6E0A - PubMed Abstract:
Bacteria use chemoreceptor proteins to sense and navigate their chemical environments. The most common class of chemoreceptors are transmembrane proteins that sense chemical cues through binding of a small-molecule ligand to a periplasmic domain, which modulates the receptor's ability to stimulate reversal of the cell's flagella motors. The prevalent gastric pathogen Helicobacter pylori uses such membrane-bound chemoreceptors, called transducer-like proteins (Tlp), to colonize and persist within the stomach. TlpA has been implicated in sensing arginine, bicarbonate, and acid, but no experimentally determined protein structures of TlpA were available to better understand ligand binding and signal transduction. Here, we report three crystal structures of the periplasmic portion of TlpA, which contains tandem PAS/Cache domains, similar to a recently published structure of the lactate-sensing chemoreceptor TlpC from H. pylori. These structures are the first to show a tandem PAS/Cache-form chemoreceptor in its native homo dimer oligomer, and we identify residues that are key contributers to the dimer interface. We performed sequence analyses to identify TlpA and TlpC homologs and used residue conservation among these homologs to implicate regions important for the general tandem PAS/Cache fold, and residues specific to TlpA function. Comparisons with TlpC show that despite high similarity across the general structure, TlpA lacks the residues required to bind lactate, and instead contains a pocket almost entirely hydrophobic in nature.
Organizational Affiliation:
Institute of Molecular Biology, University of Oregon, Eugene, Oregon, 97403.