Trypanosomatid Deoxyhypusine Synthase Activity Is Dependent on Shared Active-Site Complementation between Pseudoenzyme Paralogs.
Afanador, G.A., Tomchick, D.R., Phillips, M.A.(2018) Structure 26: 1499-1512.e5
- PubMed: 30197036
- DOI: https://doi.org/10.1016/j.str.2018.07.012
- Primary Citation of Related Structures:
6DFT - PubMed Abstract:
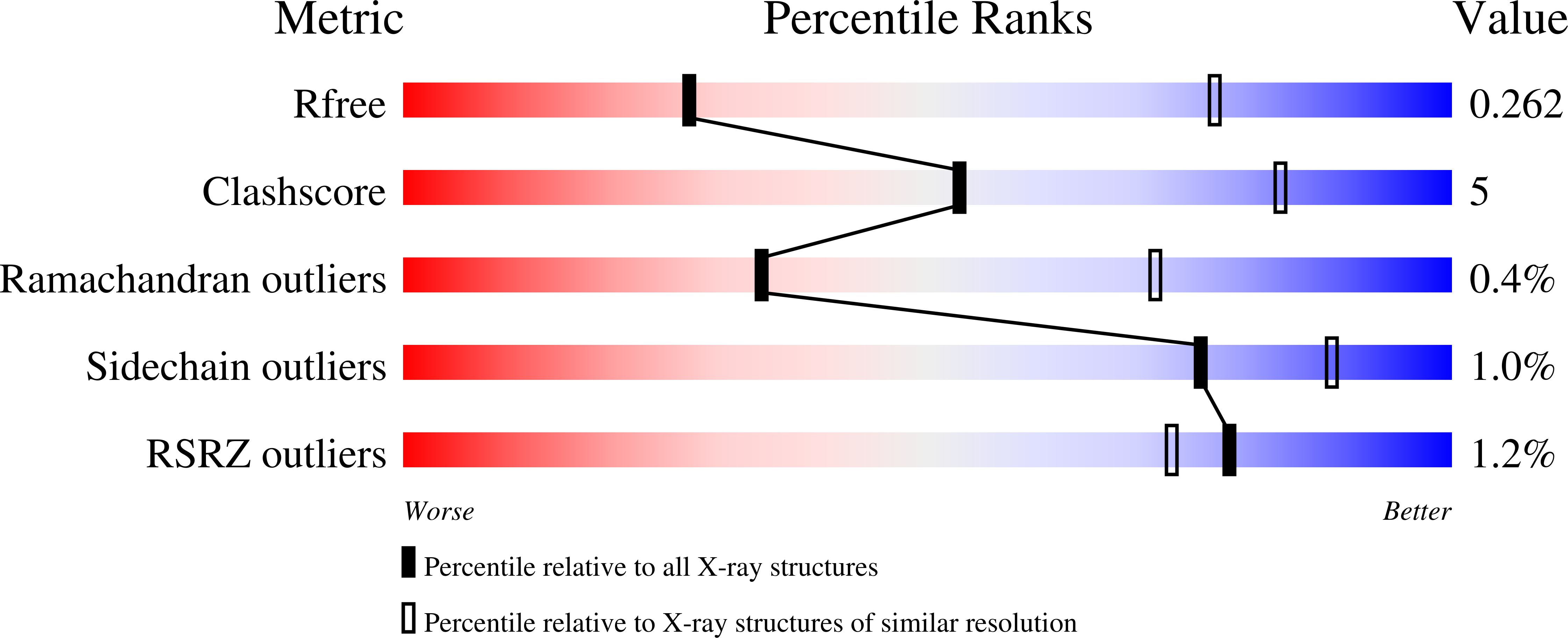
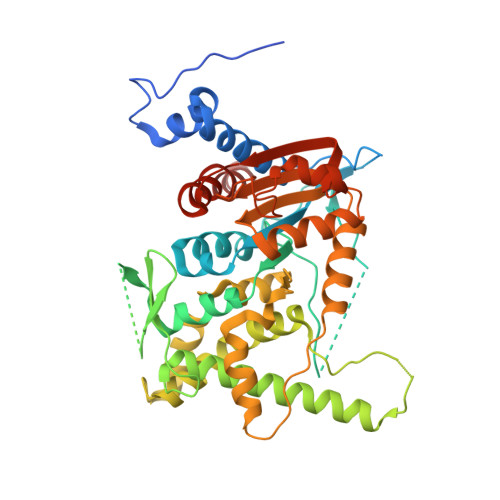
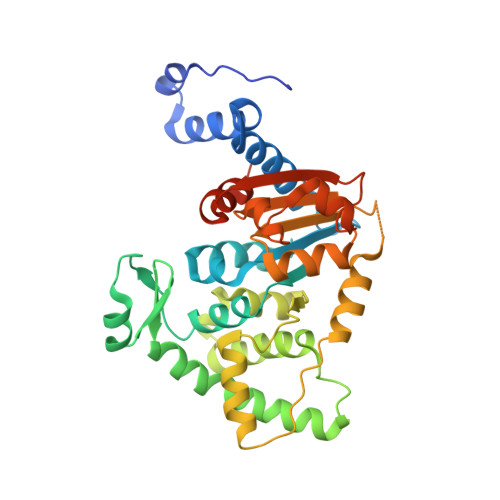
Trypanosoma brucei is a neglected tropical disease endemic to Africa. The polyamine spermidine is essential for post-translational hypusine modification of eukaryotic initiation factor 5A (eIF5A), which is catalyzed by deoxyhypusine synthase (TbDHS). In trypanosomatids, deoxyhypusine synthase (DHS) activity is dependent on heterotetramer formation between two paralogs, DHSc and DHSp, both with minimal activity on their own due to missing catalytic residues. We determined the X-ray structure of TbDHS showing a single functional shared active site is formed at the DHSc/DHSp heterodimer interface, with deficiencies in one subunit complemented by the other. Each heterodimer contains two NAD + binding sites, one housed in the functional catalytic site and the second bound in a remnant dead site that lacks key catalytic residues. Functional analysis of these sites by site-directed mutagenesis identified long-range contributions to the catalytic site from the dead site. Differences between trypanosomatid and human DHS that could be exploited for drug discovery were identified.
Organizational Affiliation:
Department of Biochemistry, University of Texas Southwestern Medical Center, Dallas, TX 75390, USA.