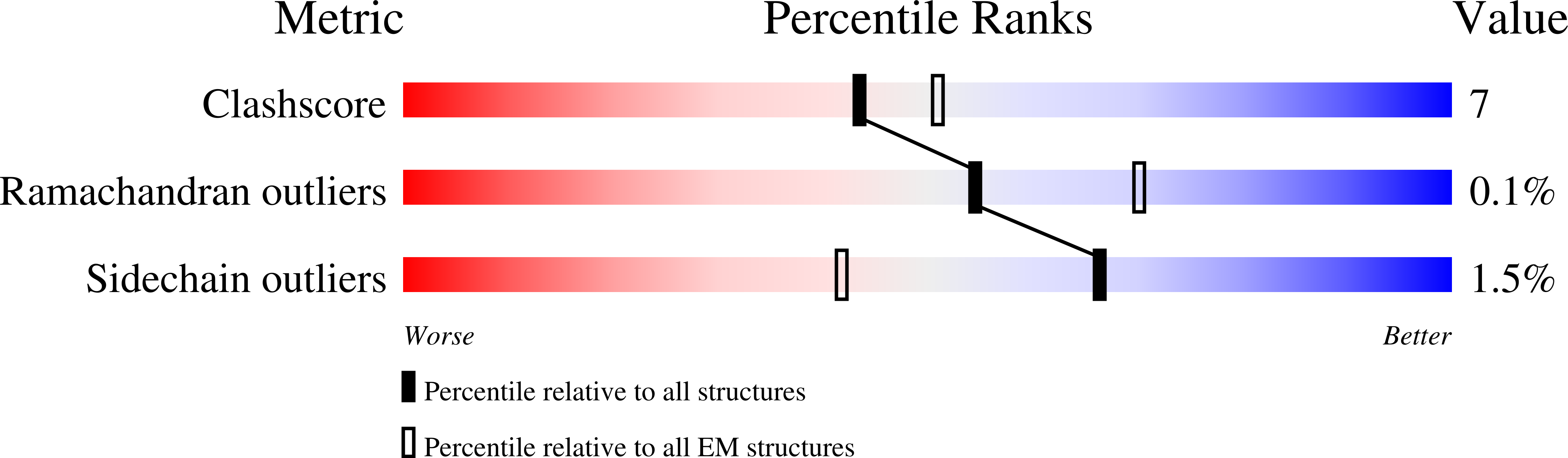Visualizing structural transitions of ligand-dependent gating of the TRPM2 channel.
Yin, Y., Wu, M., Hsu, A.L., Borschel, W.F., Borgnia, M.J., Lander, G.C., Lee, S.Y.(2019) Nat Commun 10: 3740-3740
- PubMed: 31431622
- DOI: https://doi.org/10.1038/s41467-019-11733-5
- Primary Citation of Related Structures:
6D73, 6PKV, 6PKW, 6PKX - PubMed Abstract:
The transient receptor potential melastatin 2 (TRPM2) channel plays a key role in redox sensation in many cell types. Channel activation requires binding of both ADP-ribose (ADPR) and Ca 2+ . The recently published TRPM2 structures from Danio rerio in the ligand-free and the ADPR/Ca 2+ -bound conditions represent the channel in closed and open states, which uncovered substantial tertiary and quaternary conformational rearrangements. However, it is unclear how these rearrangements are achieved within the tetrameric channel during channel gating. Here we report the cryo-electron microscopy structures of Danio rerio TRPM2 in the absence of ligands, in complex with Ca 2+ alone, and with both ADPR and Ca 2+ , resolved to ~4.3 Å, ~3.8 Å, and ~4.2 Å, respectively. In contrast to the published results, our studies capture ligand-bound TRPM2 structures in two-fold symmetric intermediate states, offering a glimpse of the structural transitions that bridge the closed and open conformations.
Organizational Affiliation:
Department of Biochemistry, Duke University School of Medicine, Durham, NC, 27710, USA.