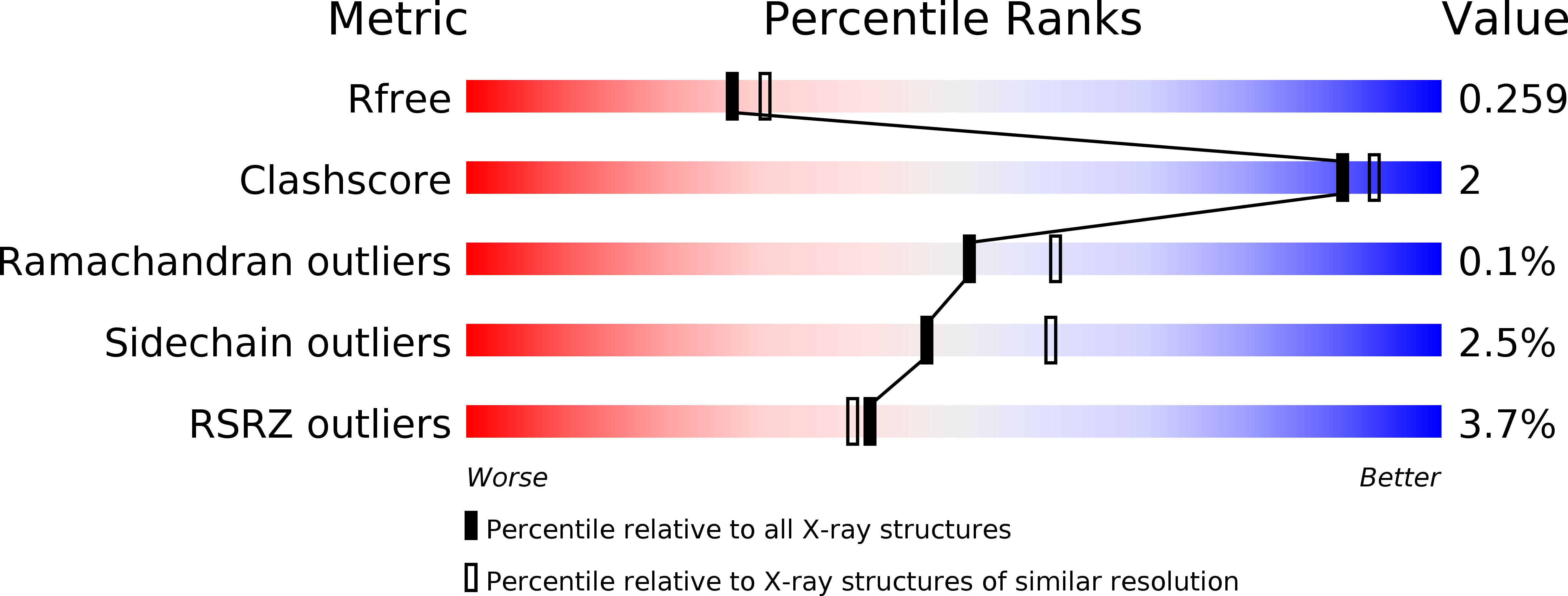
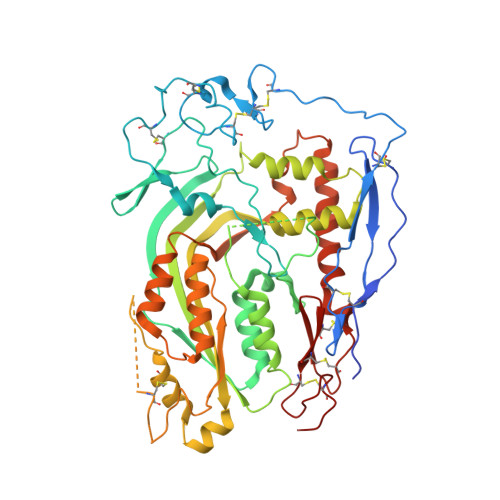
The first transmembrane region of complement component-9 acts as a brake on its self-assembly.
Spicer, B.A., Law, R.H.P., Caradoc-Davies, T.T., Ekkel, S.M., Bayly-Jones, C., Pang, S.S., Conroy, P.J., Ramm, G., Radjainia, M., Venugopal, H., Whisstock, J.C., Dunstone, M.A.(2018) Nat Commun 9: 3266-3266
- PubMed: 30111885
- DOI: https://doi.org/10.1038/s41467-018-05717-0
- Primary Citation of Related Structures:
6CXO, 6DLW - PubMed Abstract:
Complement component 9 (C9) functions as the pore-forming component of the Membrane Attack Complex (MAC). During MAC assembly, multiple copies of C9 are sequentially recruited to membrane associated C5b8 to form a pore. Here we determined the 2.2 Å crystal structure of monomeric murine C9 and the 3.9 Å resolution cryo EM structure of C9 in a polymeric assembly. Comparison with other MAC proteins reveals that the first transmembrane region (TMH1) in monomeric C9 is uniquely positioned and functions to inhibit its self-assembly in the absence of C5b8. We further show that following C9 recruitment to C5b8, a conformational change in TMH1 permits unidirectional and sequential binding of additional C9 monomers to the growing MAC. This mechanism of pore formation contrasts with related proteins, such as perforin and the cholesterol dependent cytolysins, where it is believed that pre-pore assembly occurs prior to the simultaneous release of the transmembrane regions.
Organizational Affiliation:
ARC Centre of Excellence in Advanced Molecular Imaging, Department of Biochemistry and Molecular Biology, 23 Innovation Walk, Monash University, Victoria, 3800, Australia.