Crystal Structures of Candida albicans Phosphodiesterase 2 and Implications for Its Biological Functions.
Yao, T., Huang, Y., Zhang, M., Chen, Y., Pei, H., Shi, J., Wang, H., Wang, Y., Ke, H.(2018) Biochemistry 57: 6070-6077
- PubMed: 30231198
- DOI: https://doi.org/10.1021/acs.biochem.8b00707
- Primary Citation of Related Structures:
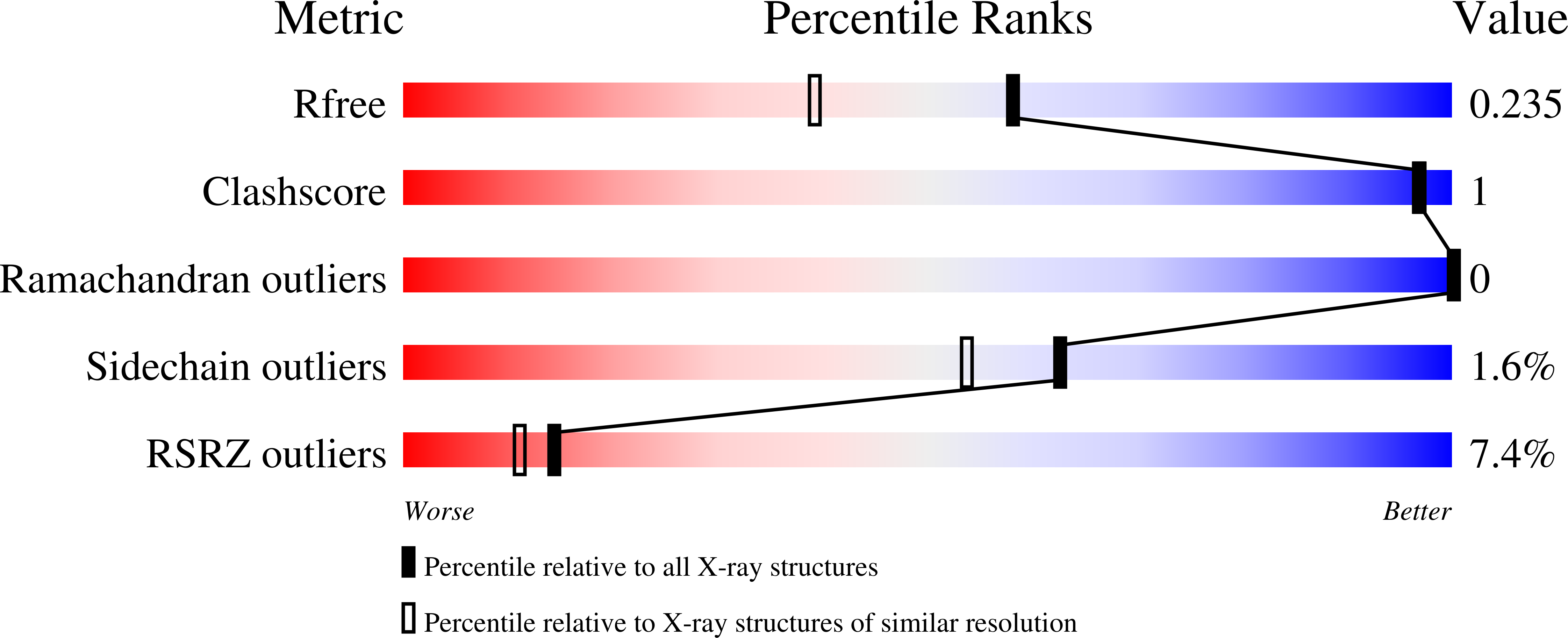
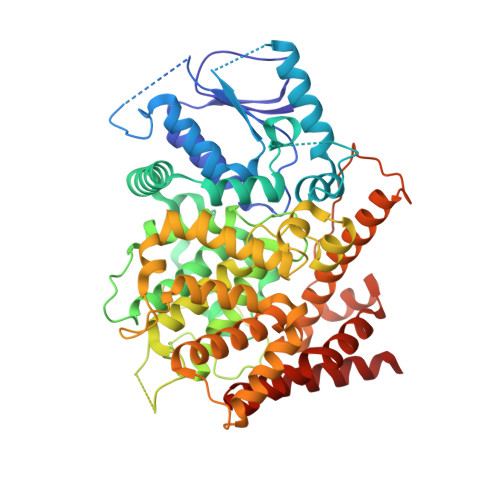
6CPT, 6CPU - PubMed Abstract:
The cAMP signaling system plays important roles in the physiological processes of pathogen yeast Candida albicans, but its functional mechanism has not been well illustrated. Here, we report the enzymatic characterization and crystal structures of C. albicans phosphodiesterase 2 (caPDE2) in the unliganded and 3-isobutyl-1-methylxanthine-complexed forms. caPDE2 is a monomer in liquid and crystal states and specifically hydrolyzes cAMP with a K M of 35 nM. It does not effectively hydrolyze cGMP as shown by the 1.32 × 10 5 -fold specificity of cAMP/cGMP. The crystal structure of caPDE2 shows significant differences from those of human PDEs. First, the N-terminal fragment of caPDE2 (residues 1-201) tightly associates with the catalytic domain to form a rigid molecular entity, implying its stable molecular conformation for C. albicans to resist environmental stresses. Second, the M-loop, a critical fragment for binding of the substrate and inhibitors to human PDEs, is not a part of the caPDE2 active site. This feature of caPDE2 may provide a structural basis for the design of selective inhibitors for the treatment of yeast infection.
Organizational Affiliation:
Beijing Advanced Innovation Center for Food Nutrition and Human Health , Beijing Technology and Business University , Beijing 100048 , China.