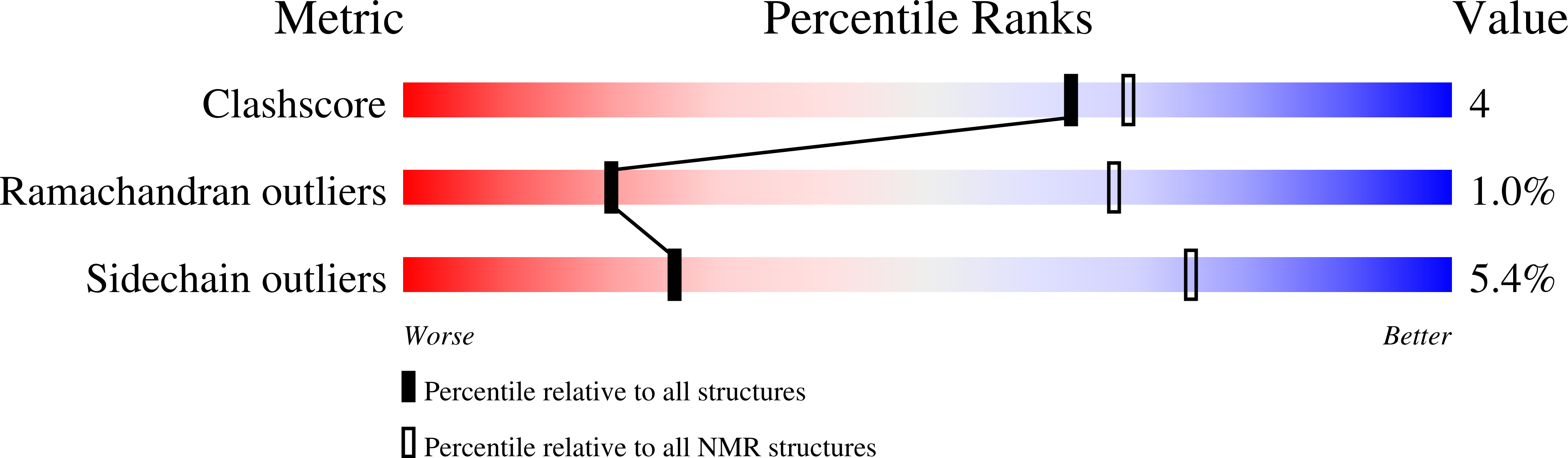Structure of the Rpn13-Rpn2 complex provides insights for Rpn13 and Uch37 as anticancer targets.
Lu, X., Nowicka, U., Sridharan, V., Liu, F., Randles, L., Hymel, D., Dyba, M., Tarasov, S.G., Tarasova, N.I., Zhao, X.Z., Hamazaki, J., Murata, S., Burke, T.R., Walters, K.J.(2017) Nat Commun 8: 15540
- PubMed: 28598414
- DOI: https://doi.org/10.1038/ncomms15540
- Primary Citation of Related Structures:
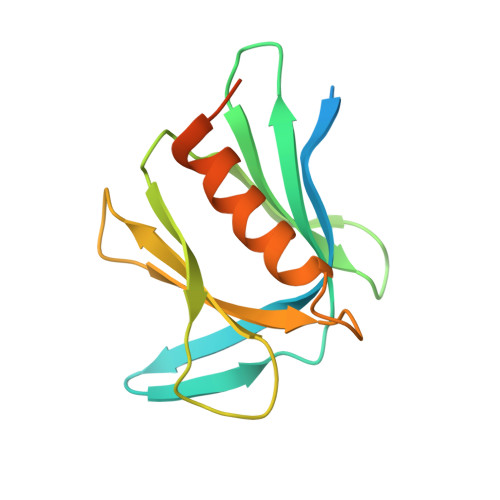
6CO4 - PubMed Abstract:
Proteasome-ubiquitin receptor hRpn13/Adrm1 binds and activates deubiquitinating enzyme Uch37/UCHL5 and is targeted by bis-benzylidine piperidone RA190, which restricts cancer growth in mice xenografts. Here, we solve the structure of hRpn13 with a segment of hRpn2 that serves as its proteasome docking site; a proline-rich C-terminal hRpn2 extension stretches across a narrow canyon of the ubiquitin-binding hRpn13 Pru domain blocking an RA190-binding surface. Biophysical analyses in combination with cell-based assays indicate that hRpn13 binds preferentially to hRpn2 and proteasomes over RA190. hRpn13 also exists outside of proteasomes where it may be RA190 sensitive. RA190 does not affect hRpn13 interaction with Uch37, but rather directly binds and inactivates Uch37. hRpn13 deletion from HCT116 cells abrogates RA190-induced accumulation of substrates at proteasomes. We propose that RA190 targets hRpn13 and Uch37 through parallel mechanisms and at proteasomes, RA190-inactivated Uch37 cannot disassemble hRpn13-bound ubiquitin chains.
Organizational Affiliation:
Protein Processing Section, Structural Biophysics Laboratory, Center for Cancer Research, National Cancer Institute, Frederick, Maryland 21702, USA.