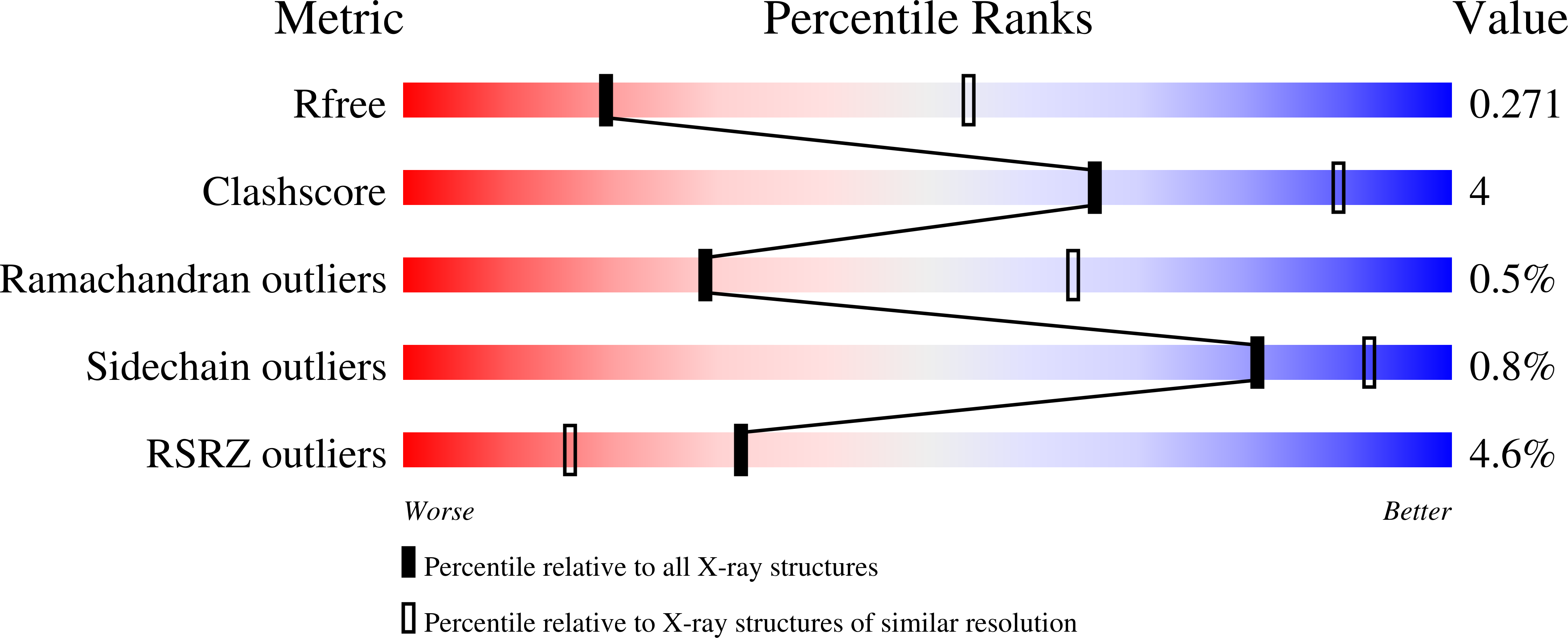
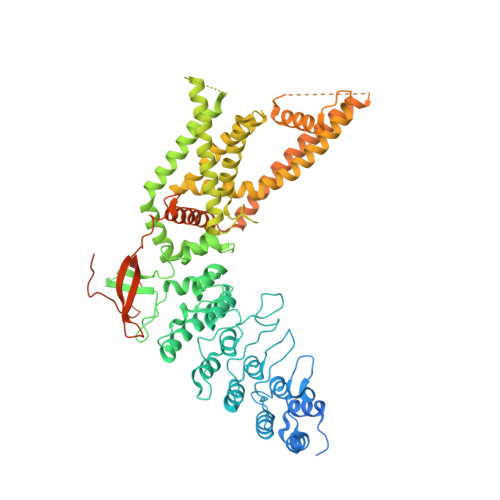
Conformational plasticity in the selectivity filter of the TRPV2 ion channel.
Zubcevic, L., Le, S., Yang, H., Lee, S.Y.(2018) Nat Struct Mol Biol 25: 405-415
- PubMed: 29728656
- DOI: https://doi.org/10.1038/s41594-018-0059-z
- Primary Citation of Related Structures:
6BWJ, 6BWM - PubMed Abstract:
Transient receptor potential vanilloid (TRPV) channels are activated by ligands and heat and are involved in various physiological processes. In contrast to the architecturally related voltage-gated cation channels, TRPV1 and TRPV2 subtypes possess another activation gate at the selectivity filter that can open widely enough to permeate large organic cations. Despite recent structural advances, the mechanism of selectivity filter gating and permeation for both metal ions and large molecules by TRPV1 or TRPV2 is not well known. Here, we determined two crystal structures of rabbit TRPV2 in its Ca 2+ -bound and resiniferatoxin (RTx)- and Ca 2+ -bound forms, to 3.9 Å and 3.1 Å, respectively. Notably, our structures show that RTx binding leads to two-fold symmetric opening of the selectivity filter of TRPV2 that is wide enough for large organic cation permeation. Combined with functional characterizations, our studies reveal a structural basis for permeation of Ca 2+ and large organic cations in TRPV2.
Organizational Affiliation:
Department of Biochemistry, Duke University School of Medicine, Durham, NC, USA.