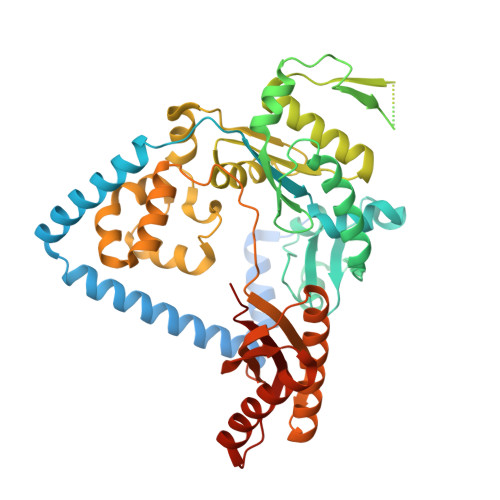
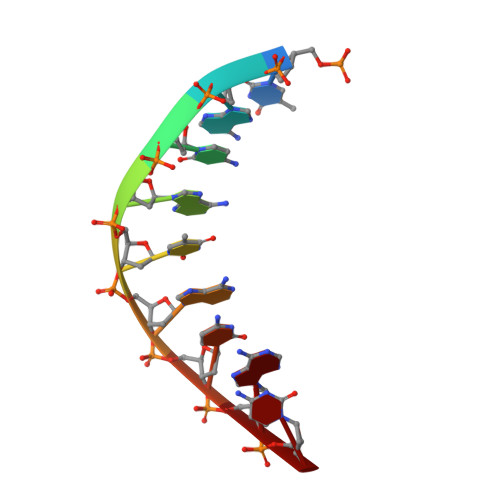
Structural Basis for Human DNA Polymerase Kappa to Bypass Cisplatin Intrastrand Cross-Link (Pt-GG) Lesion as an Efficient and Accurate Extender.
Jha, V., Ling, H.(2018) J Mol Biol 430: 1577-1589
- PubMed: 29715472
- DOI: https://doi.org/10.1016/j.jmb.2018.04.023
- Primary Citation of Related Structures:
6BRX, 6BS1 - PubMed Abstract:
Cisplatin (cis-diamminedichloroplatinum) is a common chemotherapeutic drug that reacts with the N7 atoms of adjacent guanines in DNA to form the Pt-1,2-d(GpG) intrastrand cross-link (Pt-GG), a major product to block DNA replication. Translesion DNA synthesis has been implicated in chemoresistance during cisplatin treatment of cancer due to Pt-GG lesion bypass. Gene knockdown studies in human cells have indicated a role for polκ during translesion synthesis of the Pt-GG lesion. However, the bypass activity of polκ with cisplatin lesions has not been well characterized. In this study, we investigated polκ's ability to bypass Pt-GG lesion in vitro and determined two crystal structures of polκ in complex with Pt-GG DNA. The ternary complex structures represent two consecutive stages of lesion bypass: nucleotide insertion opposite the 5'G (Pt-GG2) and primer extension immediately after the lesion (Pt-GG3). Our biochemical data showed that polκ is very efficient and accurate in extending DNA primers after the first G of the Pt-GG lesion. The structures demonstrate that the efficiency and accuracy is achieved by stably accommodating the bases with the cisplatin adduct in the active site for proper Watson-Crick base pairing with the incoming nucleotide in both the second insertion and post-insertion complexes. Our studies suggest that polκ works as an extender for efficient replication of the Pt-GG lesion in cells. This work holds promise for considering polκ, along with polη, as potential targets for drug design, which together could improve the efficacy of cisplatin treatment for cancer therapy.
Organizational Affiliation:
Department of Biochemistry, Schulich School of Medicine & Dentistry, University of Western Ontario, London, Ontario N6A 5C1, Canada.