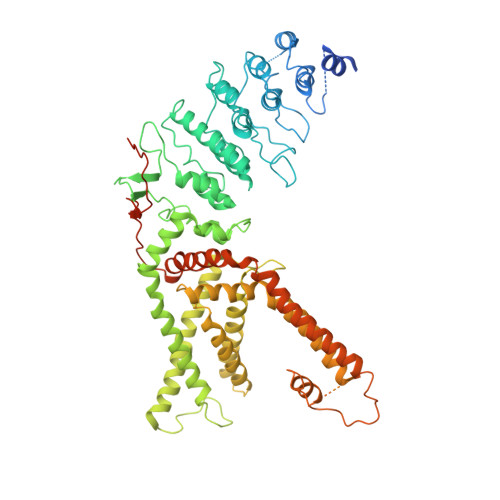
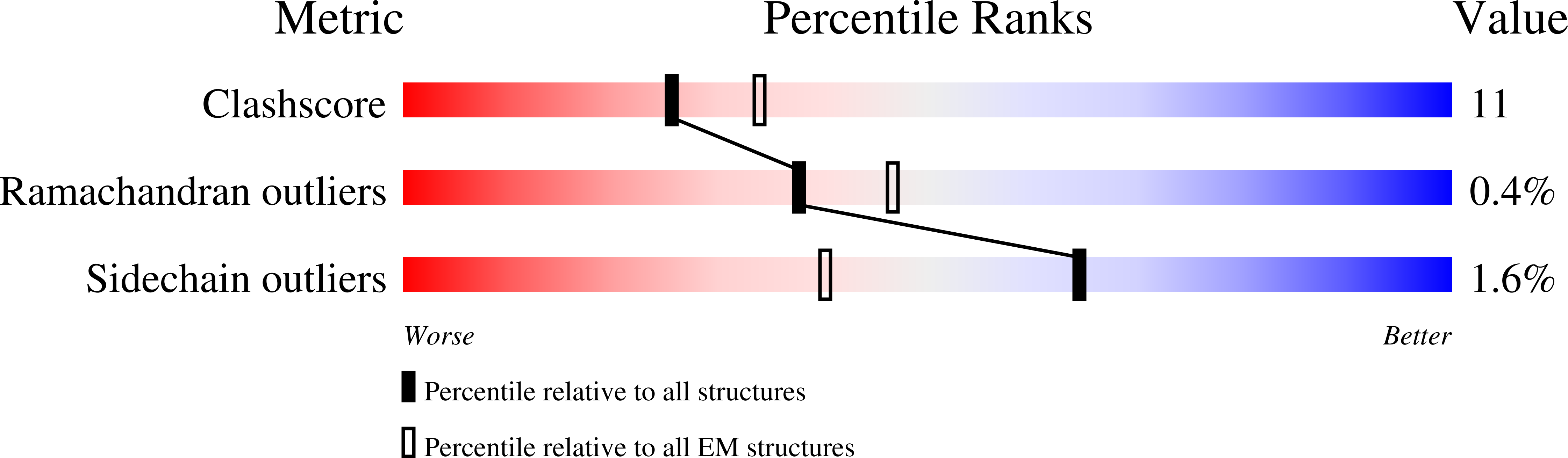Structures of TRPV2 in distinct conformations provide insight into role of the pore turret.
Dosey, T.L., Wang, Z., Fan, G., Zhang, Z., Serysheva, I.I., Chiu, W., Wensel, T.G.(2019) Nat Struct Mol Biol 26: 40-49
- PubMed: 30598551
- DOI: https://doi.org/10.1038/s41594-018-0168-8
- Primary Citation of Related Structures:
6BO4, 6BO5 - PubMed Abstract:
Cation channels of the transient receptor potential (TRP) family serve important physiological roles by opening in response to diverse intra- and extracellular stimuli that regulate their lower or upper gates. Despite extensive studies, the mechanism coupling these gates has remained obscure. Previous structures have failed to resolve extracellular loops, known in the TRPV subfamily as 'pore turrets', which are proximal to the upper gates. We established the importance of the pore turret through activity assays and by solving structures of rat TRPV2, both with and without an intact turret at resolutions of 4.0 Å and 3.6 Å, respectively. These structures resolve the full-length pore turret and reveal fully open and partially open states of TRPV2, both with unoccupied vanilloid pockets. Our results suggest a mechanism by which physiological signals, such as lipid binding, can regulate the lower gate and couple to the upper gate through a pore-turret-facilitated mechanism.
Organizational Affiliation:
Verna and Marrs Mclean Department of Biochemistry and Molecular Biology, Baylor College of Medicine, Houston, TX, USA.