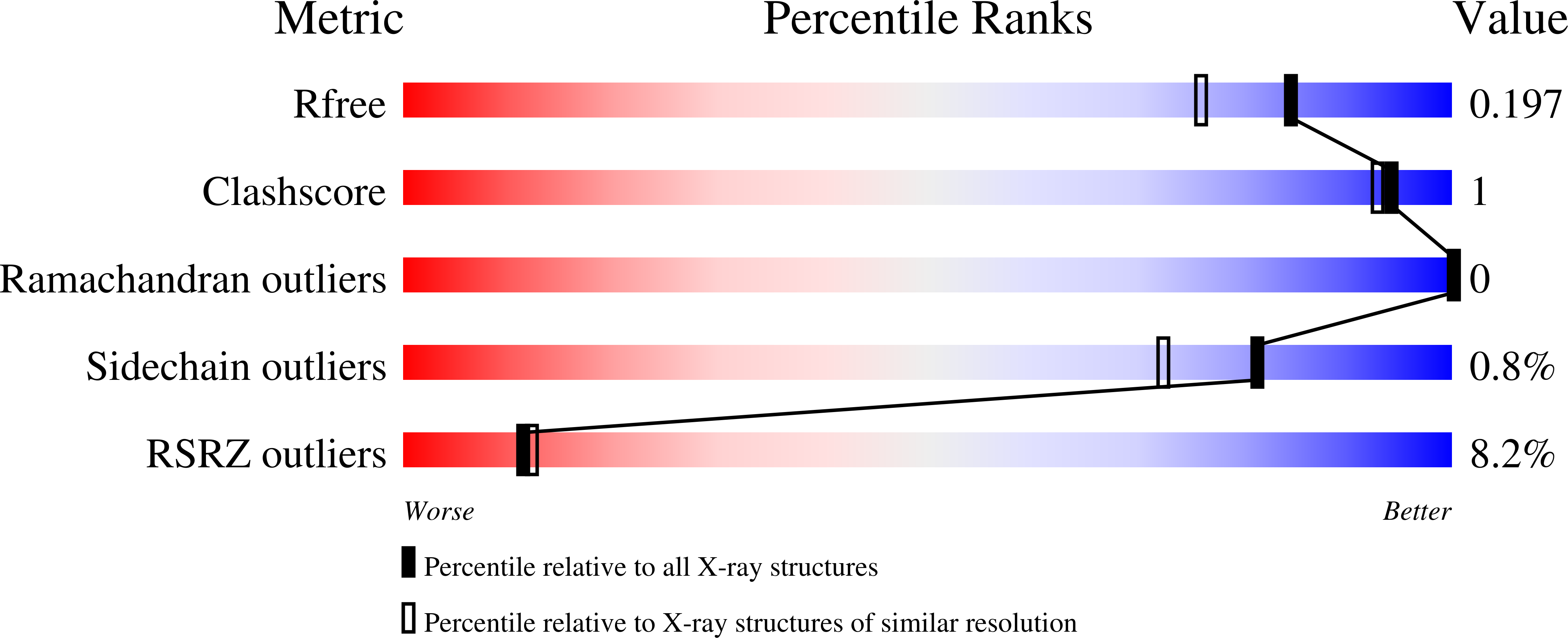
Insights into the Action of Inhibitor Enantiomers against Histone Lysine Demethylase 5A.
Horton, J.R., Liu, X., Wu, L., Zhang, K., Shanks, J., Zhang, X., Rai, G., Mott, B.T., Jansen, D.J., Kales, S.C., Henderson, M.J., Pohida, K., Fang, Y., Hu, X., Jadhav, A., Maloney, D.J., Hall, M.D., Simeonov, A., Fu, H., Vertino, P.M., Yan, Q., Cheng, X.(2018) J Med Chem 61: 3193-3208
- PubMed: 29537847
- DOI: https://doi.org/10.1021/acs.jmedchem.8b00261
- Primary Citation of Related Structures:
6BGU, 6BGV, 6BGW, 6BGX, 6BGY, 6BGZ, 6BH0, 6BH1, 6BH2, 6BH3, 6BH4, 6BH5 - PubMed Abstract:
Isomers of chiral drugs can exhibit marked differences in biological activities. We studied the binding and inhibitory activities of 12 compounds against KDM5A. Among them are two pairs of enantiomers representing two distinct inhibitor chemotypes, namely, ( R)- and ( S)-2-((2-chlorophenyl)(2-(piperidin-1-yl)ethoxy)methyl)-1 H-pyrrolo[3,2- b]pyridine-7-carboxylic acid (compounds N51 and N52) and ( R) - and ( S) -N-(1-(3-isopropyl-1 H-pyrazole-5-carbonyl)pyrrolidin-3-yl)cyclopropanecarboxamide (compounds N54 and N55). In vitro, the S enantiomer of the N51/N52 pair (N52) and the R enantiomer of the N54/N55 pair (N54) exhibited about 4- to 5-fold greater binding affinity. The more potent enzyme inhibition of KDM5A by the R-isoform for the cell-permeable N54/N55 pair translated to differences in growth inhibitory activity. We determined structures of the KDM5A catalytic domain in complex with all 12 inhibitors, which revealed the interactions (or lack thereof) responsible for the differences in binding affinity. These results provide insights to guide improvements in binding potency and avenues for development of cell permeable inhibitors of the KDM5 family.
Organizational Affiliation:
Department of Molecular and Cellular Oncology , The University of Texas MD Anderson Cancer Center , Houston , Texas 77030 , United States.