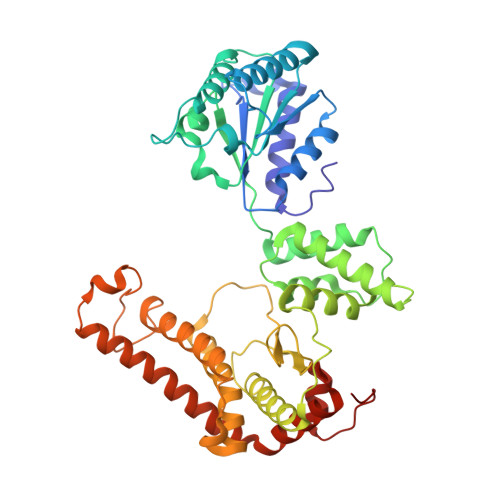
Structure of the mitochondrial inner membrane AAA+ protease YME1 gives insight into substrate processing.
Puchades, C., Rampello, A.J., Shin, M., Giuliano, C.J., Wiseman, R.L., Glynn, S.E., Lander, G.C.(2017) Science 358
- PubMed: 29097521
- DOI: https://doi.org/10.1126/science.aao0464
- Primary Citation of Related Structures:
6AZ0 - PubMed Abstract:
We present an atomic model of a substrate-bound inner mitochondrial membrane AAA+ quality control protease in yeast, YME1. Our ~3.4-angstrom cryo-electron microscopy structure reveals how the adenosine triphosphatases (ATPases) form a closed spiral staircase encircling an unfolded substrate, directing it toward the flat, symmetric protease ring. Three coexisting nucleotide states allosterically induce distinct positioning of tyrosines in the central channel, resulting in substrate engagement and translocation to the negatively charged proteolytic chamber. This tight coordination by a network of conserved residues defines a sequential, around-the-ring adenosine triphosphate hydrolysis cycle that results in stepwise substrate translocation. A hingelike linker accommodates the large-scale nucleotide-driven motions of the ATPase spiral relative to the planar proteolytic base. The translocation mechanism is likely conserved for other AAA+ ATPases.
Organizational Affiliation:
Department of Integrative Structural and Computational Biology, The Scripps Research Institute HZ 175, 10550 North Torrey Pines Road, La Jolla, CA 92037, USA.