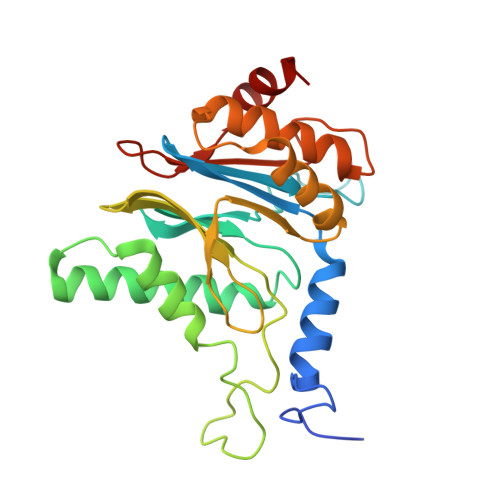
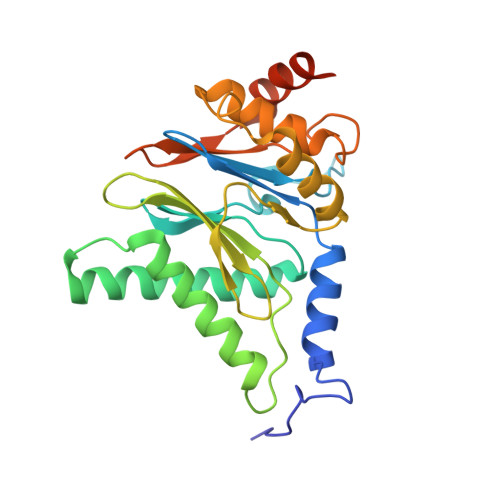
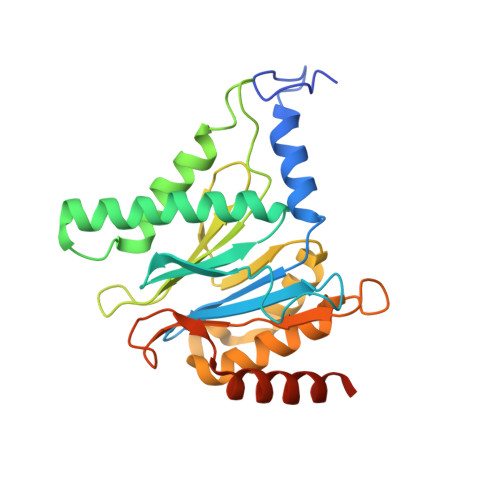
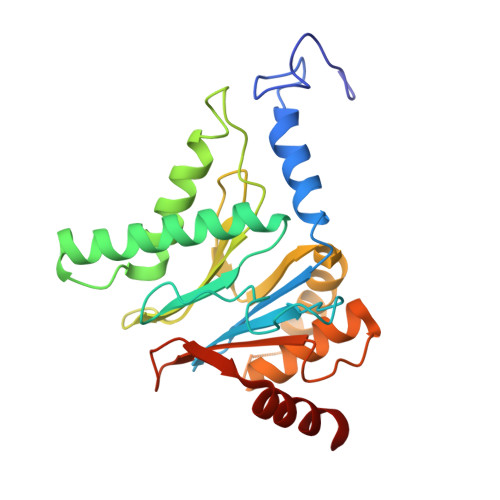
Structure of human immunoproteasome with a reversible and noncompetitive inhibitor that selectively inhibits activated lymphocytes.
Santos, R.L.A., Bai, L., Singh, P.K., Murakami, N., Fan, H., Zhan, W., Zhu, Y., Jiang, X., Zhang, K., Assker, J.P., Nathan, C.F., Li, H., Azzi, J., Lin, G.(2017) Nat Commun 8: 1692-1692
- PubMed: 29167449
- DOI: https://doi.org/10.1038/s41467-017-01760-5
- Primary Citation of Related Structures:
6AVO - PubMed Abstract:
Proteasome inhibitors benefit patients with multiple myeloma and B cell-dependent autoimmune disorders but exert toxicity from inhibition of proteasomes in other cells. Toxicity should be minimized by reversible inhibition of the immunoproteasome β5i subunit while sparing the constitutive β5c subunit. Here we report β5i-selective inhibition by asparagine-ethylenediamine (AsnEDA)-based compounds and present the high-resolution cryo-EM structural analysis of the human immunoproteasome. Despite inhibiting noncompetitively, an AsnEDA inhibitor binds the active site. Hydrophobic interactions are accompanied by hydrogen bonding with β5i and β6 subunits. The inhibitors are far more cytotoxic for myeloma and lymphoma cell lines than for hepatocarcinoma or non-activated lymphocytes. They block human B-cell proliferation and promote apoptotic cell death selectively in antibody-secreting B cells, and to a lesser extent in activated human T cells. Reversible, β5i-selective inhibitors may be useful for treatment of diseases involving activated or neoplastic B cells or activated T cells.
Organizational Affiliation:
Cryo-EM Structural Biology Laboratory, Van Andel Research Institute, Grand Rapids, MI, 49503, USA.