Untying a Protein Knot by Circular Permutation.
Chuang, Y.C., Hu, I.C., Lyu, P.C., Hsu, S.D.(2019) J Mol Biol 431: 857-863
- PubMed: 30639189
- DOI: https://doi.org/10.1016/j.jmb.2019.01.005
- Primary Citation of Related Structures:
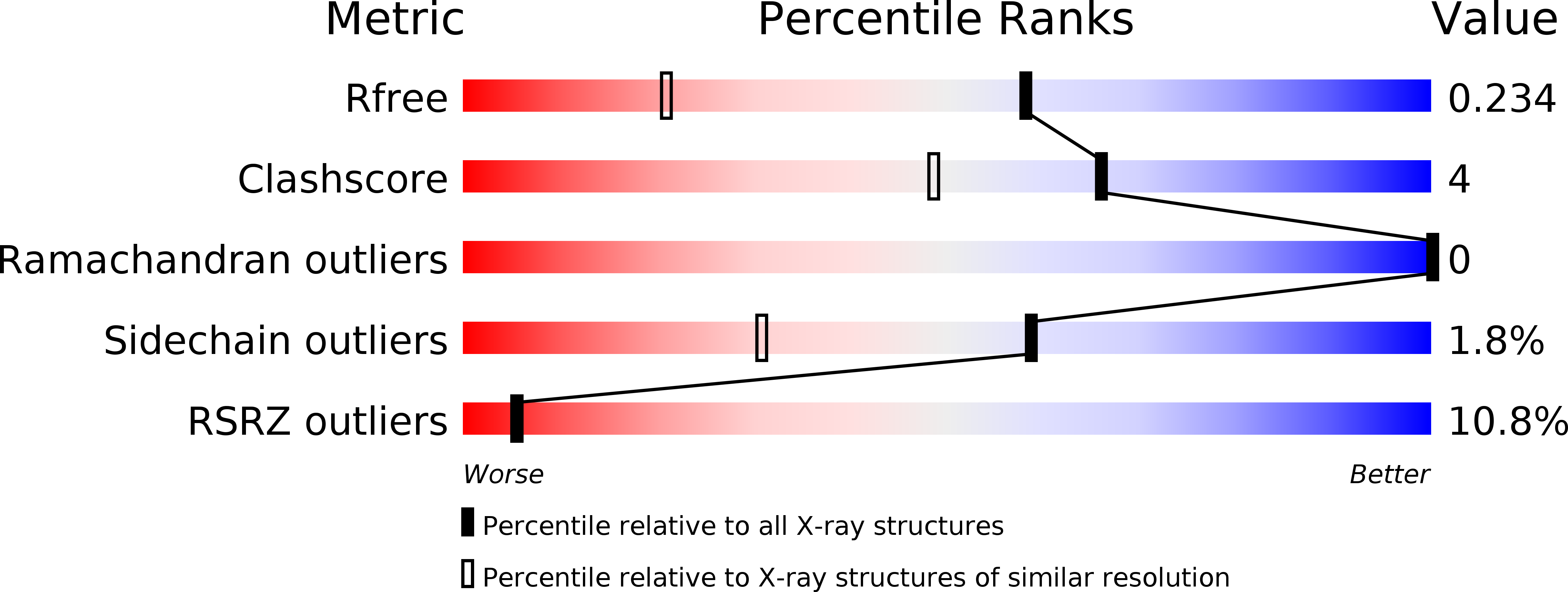
6AHW - PubMed Abstract:
Topologically knotted proteins are tantalizing examples of how polypeptide chains can explore complex free energy landscapes to efficiently attain defined knotted conformations. The evolution trails of protein knots, however, remain elusive. We used circular permutation to change an evolutionally conserved topologically knotted SPOUT RNA methyltransferase into an unknotted form. The unknotted variant adopted the same three-dimensional structure and oligomeric state as its knotted parent, but its folding stability was markedly reduced with accelerated folding kinetics and its ligand binding was abrogated. Our findings support the hypothesis that the universally conserved knotted topology of the SPOUT superfamily evolved from unknotted forms through circular permutation under selection pressure for folding robustness and, more importantly, for functional requirements associated with the knotted structural element.
Organizational Affiliation:
Institute of Biological Chemistry, Academia Sinica, Taipei 11529, Taiwan; Institute of Bioinformatics and Structural Biology, National Tsing Hua University, Hsinchu 30013, Taiwan.