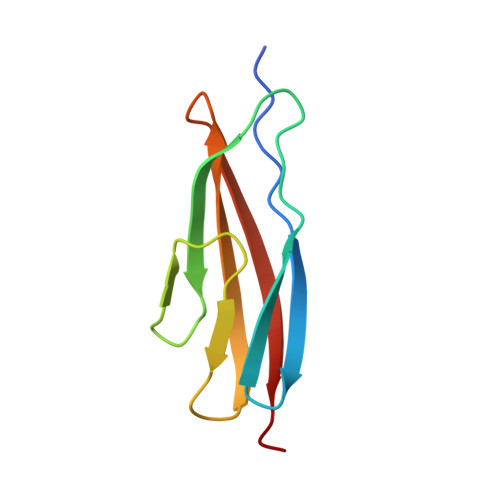
Structural basis for specific calcium binding by the polycystic-kidney-disease domain of Vibrio anguillarum protease Epp
Li, P., Zang, K., Li, Y., Liu, C., Ma, Q.(2018) Biochem Biophys Res Commun 505: 471-477
- PubMed: 30268503
- DOI: https://doi.org/10.1016/j.bbrc.2018.09.108
- Primary Citation of Related Structures:
6AEM - PubMed Abstract:
Extracellular proteases are often produced as pre-pro-enzyme and then undergo multiple processing steps to mature into the active form. The protease Epp, a virulent factor of Vibrio anguillarum, belongs to this family. Its maturation might be regulated by Ca 2+ via its polycystic kidney disease (PKD) domain, but the molecular mechanism is unknown. Herein, we report the crystal structure of the first PKD domain from V. anguillarum Epp (Epp-PKD1) and its specific Ca 2+ -binding capacity. Epp-PKD1 exists as a monomer, consisting of seven β-strands which form two β-sheets stacking with each other. One Ca 2+ is bound by the residues Asn3, Gln4, Asp27, Asp29, Asp68 and a water molecule with a pentagonal bipyramidal geometry. Incubating the apo Epp-PKD1 with Ca 2+ but not Mg 2+ , Mn 2+ , or Zn 2+ , enhances the thermal and chemical stability of Epp-PKD1, indicating its specific binding to Ca 2+ . Epp-PKD1 shares high similarity in both sequence and overall structure with that of Vibrio cholerae PrtV, a homologous protease of Epp, however, they differ in the oligomeric state and local structure at the Ca 2+ -binding site, suggesting maturation of PrtV and Epp might be differently regulated by Ca 2+ . Likely, proteases may take advantage of the structural diversity in PKD domains to tune their Ca 2+ -regulated maturation process.
Organizational Affiliation:
Key Laboratory of Experimental Marine Biology, Institute of Oceanology, Chinese Academy of Sciences, Qingdao, China; Laboratory for Marine Biology and Biotechnology, Qingdao National Laboratory for Marine Science and Technology, Qingdao, China; University of Chinese Academy of Sciences, Beijing, China.