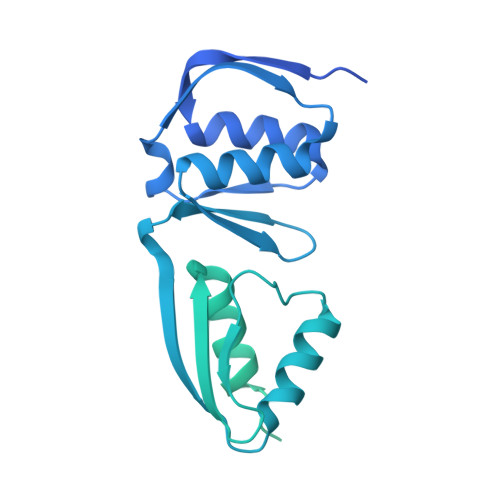
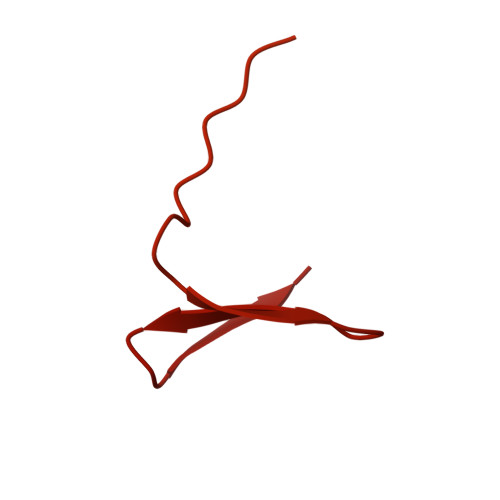
Cryo-EM structure of the Shigella type III needle complex.
Lunelli, M., Kamprad, A., Burger, J., Mielke, T., Spahn, C.M.T., Kolbe, M.(2020) PLoS Pathog 16: e1008263-e1008263
- PubMed: 32092125
- DOI: https://doi.org/10.1371/journal.ppat.1008263
- Primary Citation of Related Structures:
6RWK, 6RWX, 6RWY - PubMed Abstract:
The Type III Secretion Systems (T3SS) needle complex is a conserved syringe-shaped protein translocation nanomachine with a mass of about 3.5 MDa essential for the survival and virulence of many Gram-negative bacterial pathogens. This system is composed of a membrane-embedded basal body and an extracellular needle that deliver effector proteins into host cells. High-resolution structures of the T3SS from different organisms and infection stages are needed to understand the underlying molecular mechanisms of effector translocation. Here, we present the cryo-electron microscopy structure of the isolated Shigella T3SS needle complex. The inner membrane (IM) region of the basal body adopts 24-fold rotational symmetry and forms a channel system that connects the bacterial periplasm with the export apparatus cage. The secretin oligomer adopts a heterogeneous architecture with 16- and 15-fold cyclic symmetry in the periplasmic N-terminal connector and C-terminal outer membrane ring, respectively. Two out of three IM subunits bind the secretin connector via a β-sheet augmentation. The cryo-EM map also reveals the helical architecture of the export apparatus core, the inner rod, the needle and their intervening interfaces.
Organizational Affiliation:
Department of Structural Infection Biology, Centre for Structural Systems Biology (CSSB), Helmholtz-Centre for Infection Research (HZI), Hamburg, Germany.