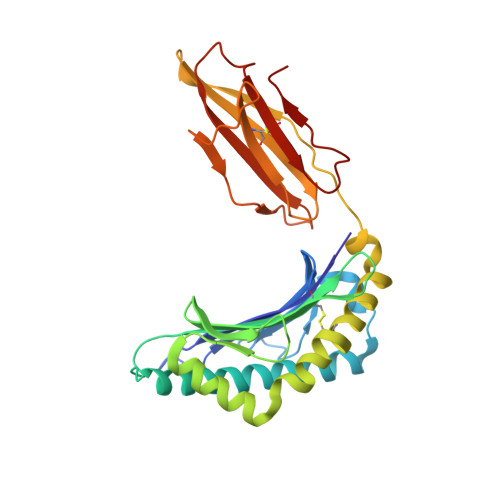
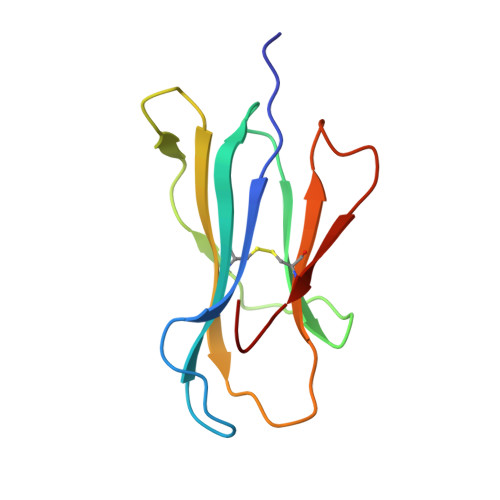
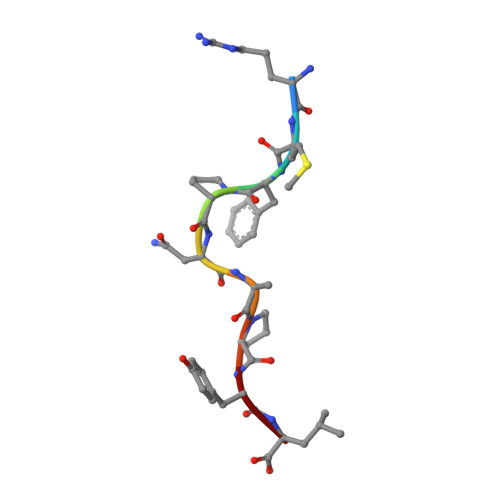
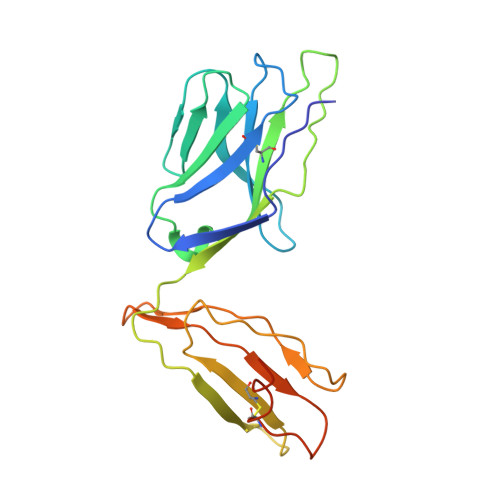
Specificity of bispecific T cell receptors and antibodies targeting peptide-HLA.
Holland, C.J., Crean, R.M., Pentier, J.M., de Wet, B., Lloyd, A., Srikannathasan, V., Lissin, N., Lloyd, K.A., Blicher, T.H., Conroy, P.J., Hock, M., Pengelly, R.J., Spinner, T.E., Cameron, B., Potter, E.A., Jeyanthan, A., Molloy, P.E., Sami, M., Aleksic, M., Liddy, N., Robinson, R.A., Harper, S., Lepore, M., Pudney, C.R., van der Kamp, M.W., Rizkallah, P.J., Jakobsen, B.K., Vuidepot, A., Cole, D.K.(2020) J Clin Invest 130: 2673-2688
- PubMed: 32310221
- DOI: https://doi.org/10.1172/JCI130562
- Primary Citation of Related Structures:
6RSY - PubMed Abstract:
Tumor-associated peptide-human leukocyte antigen complexes (pHLAs) represent the largest pool of cell surface-expressed cancer-specific epitopes, making them attractive targets for cancer therapies. Soluble bispecific molecules that incorporate an anti-CD3 effector function are being developed to redirect T cells against these targets using 2 different approaches. The first achieves pHLA recognition via affinity-enhanced versions of natural TCRs (e.g., immune-mobilizing monoclonal T cell receptors against cancer [ImmTAC] molecules), whereas the second harnesses an antibody-based format (TCR-mimic antibodies). For both classes of reagent, target specificity is vital, considering the vast universe of potential pHLA molecules that can be presented on healthy cells. Here, we made use of structural, biochemical, and computational approaches to investigate the molecular rules underpinning the reactivity patterns of pHLA-targeting bispecifics. We demonstrate that affinity-enhanced TCRs engage pHLA using a comparatively broad and balanced energetic footprint, with interactions distributed over several HLA and peptide side chains. As ImmTAC molecules, these TCRs also retained a greater degree of pHLA selectivity, with less off-target activity in cellular assays. Conversely, TCR-mimic antibodies tended to exhibit binding modes focused more toward hot spots on the HLA surface and exhibited a greater degree of crossreactivity. Our findings extend our understanding of the basic principles that underpin pHLA selectivity and exemplify a number of molecular approaches that can be used to probe the specificity of pHLA-targeting molecules, aiding the development of future reagents.
Organizational Affiliation:
Immunocore Ltd., Milton Park, Abingdon, United Kingdom.