Structure of mouse CD1D- Glc-DAG (sn-1 C18:0, sn-2 C18:1c9)-iNKT TCR Ternary complex
Dirk, M.Z., Bitra, A., Wang, J.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Currently 6OMG does not have a validation slider image.
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| CHIMERIC T CELL ANTIGEN RECEPTOR ALPHA CHAIN VA14,VA24 | A [auth C] | 209 | Mus musculus | Mutation(s): 0 | 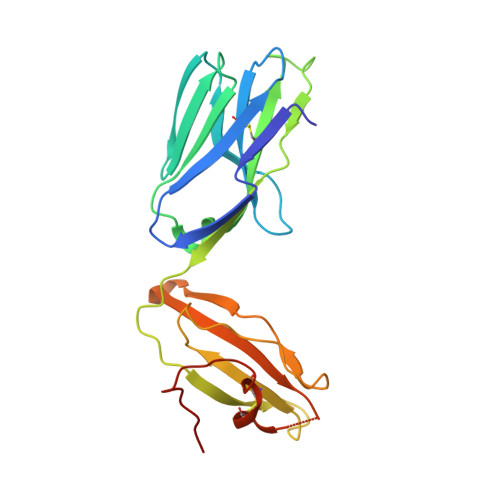 |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| CHIMERIC T CELL ANTIGEN RECEPTOR BETA CHAIN VB8.2, VB11 | B [auth D] | 241 | Mus musculus | Mutation(s): 0 | 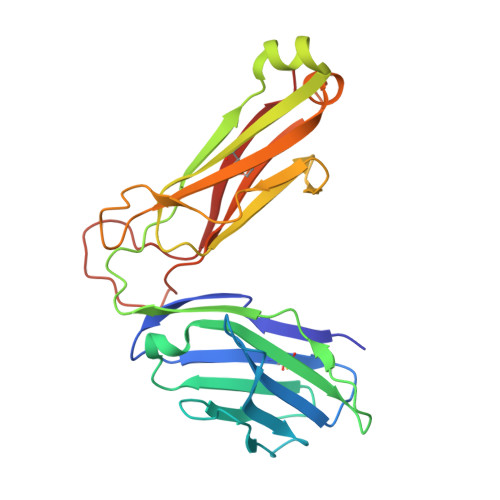 |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Antigen-presenting glycoprotein CD1d1 | C [auth A] | 285 | Mus musculus | Mutation(s): 0 Gene Names: Cd1d1, mCG_3074 | 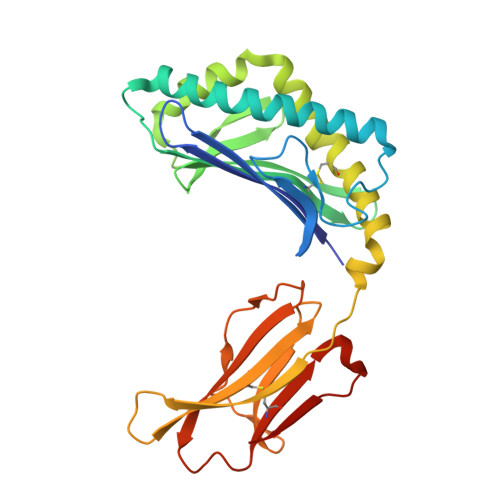 |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P11609 (Mus musculus) Explore P11609 Go to UniProtKB: P11609 | |||||
IMPC: MGI:107674 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P11609 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 4 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Beta-2-microglobulin | D [auth B] | 99 | Mus musculus | Mutation(s): 0 Gene Names: B2m | 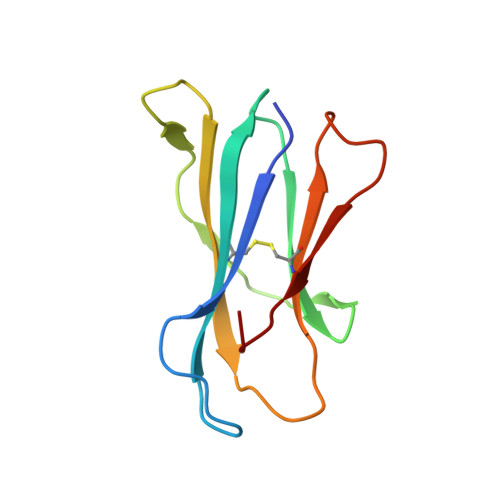 |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P01887 (Mus musculus) Explore P01887 Go to UniProtKB: P01887 | |||||
IMPC: MGI:88127 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P01887 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| MVV Query on MVV | N [auth A] | (2R)-1-(alpha-D-glucopyranosyloxy)-3-(octadecanoyloxy)propan-2-yl (9Z)-octadec-9-enoate C45 H84 O10 STIYDDOYHOERHV-JTDQAXPSSA-N |  | ||
| GOL Query on GOL | H [auth C], I [auth C], L [auth A], M [auth A] | GLYCEROL C3 H8 O3 PEDCQBHIVMGVHV-UHFFFAOYSA-N |  | ||
| NA Query on NA | J [auth C], K [auth D], O [auth A], P [auth A], Q [auth A] | SODIUM ION Na FKNQFGJONOIPTF-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 78.692 | α = 90 |
| b = 191.25 | β = 90 |
| c = 151.29 | γ = 90 |
| Software Name | Purpose |
|---|---|
| HKL-2000 | data scaling |
| REFMAC | refinement |
| PDB_EXTRACT | data extraction |
| HKL-2000 | data reduction |
| PHASER | phasing |
Currently 6OMG does not have a validation slider image.