Cross-Reactive Donor-Specific CD8+Tregs Efficiently Prevent Transplant Rejection.
Picarda, E., Bezie, S., Usero, L., Ossart, J., Besnard, M., Halim, H., Echasserieau, K., Usal, C., Rossjohn, J., Bernardeau, K., Gras, S., Guillonneau, C.(2019) Cell Rep 29: 4245-4255.e6
- PubMed: 31875536
- DOI: https://doi.org/10.1016/j.celrep.2019.11.106
- Primary Citation of Related Structures:
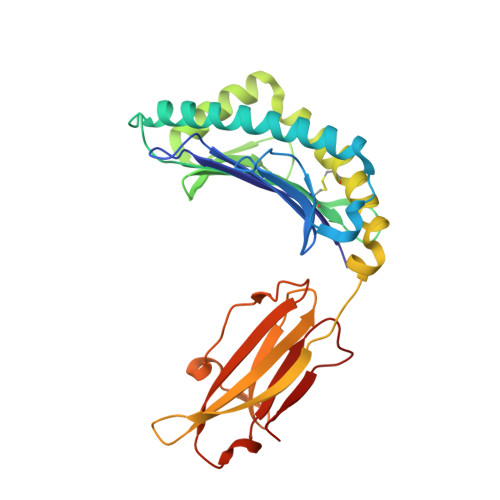
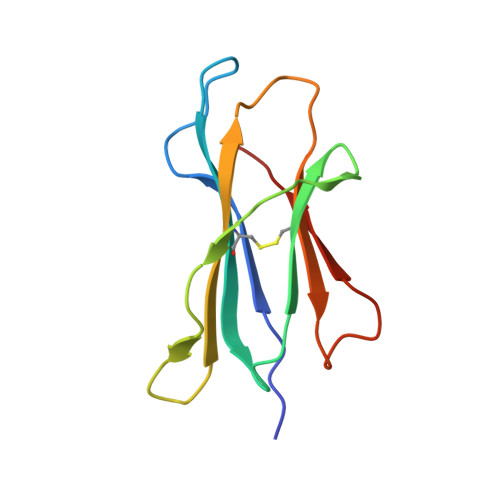
6NF7 - PubMed Abstract:
To reduce the use of non-specific immunosuppressive drugs detrimental to transplant patient health, therapies in development aim to achieve antigen-specific tolerance by promoting antigen-specific regulatory T cells (Tregs). However, identification of the natural antigens recognized by Tregs and the contribution of their dominance in transplantation has been challenging. We identify epitopes derived from distinct major histocompatibility complex (MHC) class II molecules, sharing a 7-amino acid consensus sequence positioned in a central mobile section in complex with MHC class I, recognized by cross-reactive CD8 + Tregs, enriched in the graft. Antigen-specific CD8 + Tregs can be induced in vivo with a 16-amino acid-long peptide to trigger transplant tolerance. Peptides derived from human HLA class II molecules, harboring the rat consensus sequence, also activate and expand human CD8 + Tregs, suggesting its potential in human transplantation. Altogether, this work should facilitate the development of therapies with peptide epitopes for transplantation and improve our understanding of CD8 + Treg recognition.
Organizational Affiliation:
Nantes Université, INSERM, Centre de Recherche en Transplantation et Immunologie, UMR 1064, ITUN, 44000 Nantes, France; LabEx IGO "Immunotherapy, Graft, Oncology," Nantes, France.