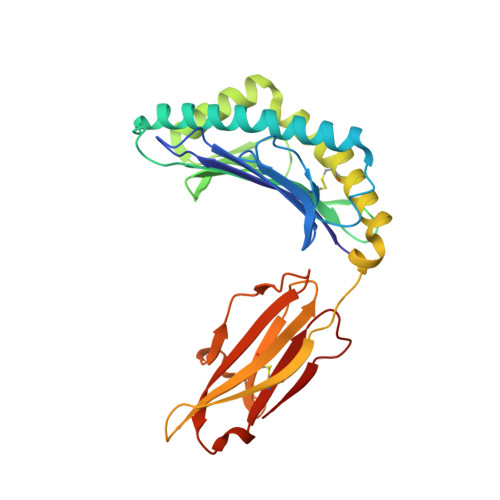
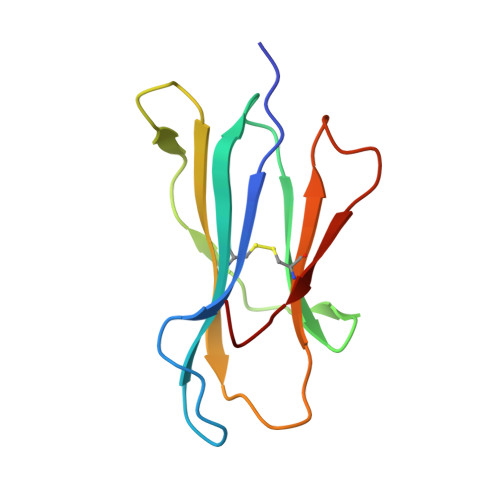
Salt bridge-forming residues positioned over viral peptides presented by MHC class I impacts T-cell recognition in a binding-dependent manner.
Ji, W., Niu, L., Peng, W., Zhang, Y., Cheng, H., Gao, F., Shi, Y., Qi, J., Gao, G.F., Liu, W.J.(2019) Mol Immunol 112: 274-282
- PubMed: 31226552
- DOI: https://doi.org/10.1016/j.molimm.2019.06.005
- Primary Citation of Related Structures:
6IEX - PubMed Abstract:
The viral peptides presentation by major histocompatibility complex class I (MHC I) molecules play a pivotal role in T-cell recognition and the subsequent virus clearance. This process is delicately adjusted by the variant residues of MHC I, especially the residues in the peptide binding groove (PBG). In a series of MHC I molecules, a salt bridge is formed above the N-terminus of the peptides. However, the potential impact of the salt bridge on peptide binding and T-cell receptor (TCR) recognition of MHC I, as well as the corresponding molecular basis, are still largely unknown. Herein, we determined the structures of HLA-B*4001 and H-2K d in which two different types of salt bridges (Arg62-Glu163 or Arg66-Glu163) across the PBG were observed. Although the two salt bridges led to different conformation shifts of both the MHC I α helix and the peptides, binding of the peptides with the salt bridge residues was relatively conserved. Furthermore, through a series of in vitro and in vivo investigations, we found that MHC I mutations that disrupt the salt bridge alleviate peptide binding and can weaken the TCR recognition of MHC I-peptide complexes. Our study may provide key references for understanding MHC I-restricted peptide recognition by T-cells.
Organizational Affiliation:
NHC Key Laboratory of Medical Virology and Viral Diseases, National Institute for Viral Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing 100052, China.