Structure-Guided Combinatorial Engineering Facilitates Affinity and Specificity Optimization of Anti-CD81 Antibodies.
Nelson, B., Adams, J., Kuglstatter, A., Li, Z., Harris, S.F., Liu, Y., Bohini, S., Ma, H., Klumpp, K., Gao, J., Sidhu, S.S.(2018) J Mol Biol 430: 2139-2152
- PubMed: 29778602
- DOI: https://doi.org/10.1016/j.jmb.2018.05.018
- Primary Citation of Related Structures:
6EJG, 6EJM, 6EK2 - PubMed Abstract:
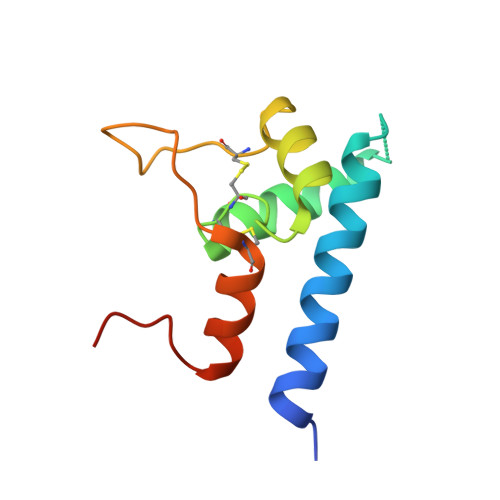
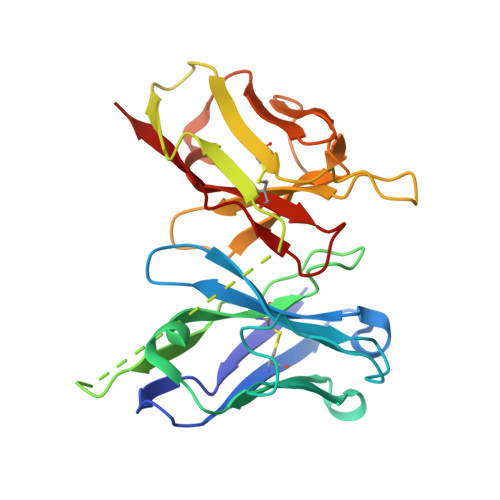
Hepatitis C viral infection is the major cause of chronic hepatitis that affects as many as 71 million people worldwide. Rather than target the rapidly shifting viruses and their numerous serotypes, four independent antibodies were made to target the host antigen CD81 and were shown to block hepatitis C viral entry. The single-chain variable fragment of each antibody was crystallized in complex with the CD81 large extracellular loop in order to guide affinity maturation of two distinct antibodies by phage display. Affinity maturation of antibodies using phage display has proven to be critical to therapeutic antibody development and typically involves modification of the paratope for increased affinity, improved specificity, enhanced stability or a combination of these traits. One antibody was engineered for increased affinity for human CD81 large extracellular loop that equated to increased efficacy, while the second antibody was engineered for cross-reactivity with cynomolgus CD81 to facilitate animal model testing. The use of structures to guide affinity maturation library design demonstrates the utility of combining structural analysis with phage display technologies.
Organizational Affiliation:
Banting and Best Department of Medical Research and Department of Medical Genetics, The Donnelly Centre for Cellular and Biomolecular Research, University of Toronto, 160 College Street, Toronto, ON M5S 3E1, Canada.