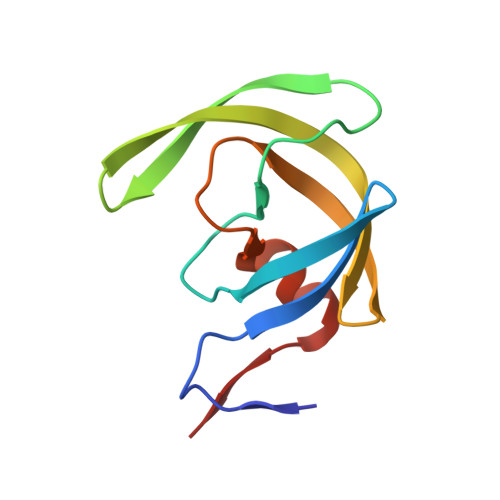
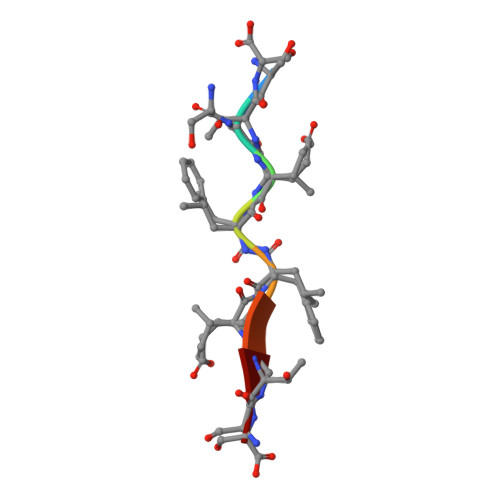
A substrate selected by phage display exhibits enhanced side-chain hydrogen bonding to HIV-1 protease.
Windsor, I.W., Raines, R.T.(2018) Acta Crystallogr D Struct Biol 74: 690-694
- PubMed: 29968678
- DOI: https://doi.org/10.1107/S2059798318006691
- Primary Citation of Related Structures:
6BRA - PubMed Abstract:
Crystal structures of inactive variants of HIV-1 protease bound to peptides have revealed how the enzyme recognizes its endogenous substrates. The best of the known substrates is, however, a nonnatural substrate that was identified by directed evolution. The crystal structure of the complex between this substrate and the D25N variant of the protease is reported at a resolution of 1.1 Å. The structure has several unprecedented features, especially the formation of additional hydrogen bonds between the enzyme and the substrate. This work expands the understanding of molecular recognition by HIV-1 protease and informs the design of new substrates and inhibitors.
Organizational Affiliation:
Department of Chemistry, Massachusetts Institute of Technology, Cambridge, MA 02139, USA.