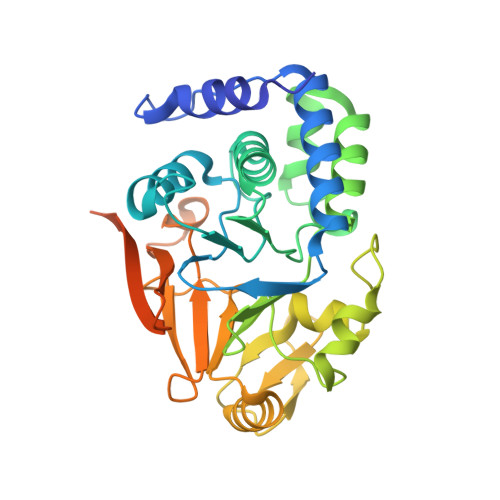
Structural basis for protein phosphatase 1 recruitment by glycogen-targeting subunits.
Yu, J., Deng, T., Xiang, S.(2018) FEBS J 285: 4646-4659
- PubMed: 30422398
- DOI: https://doi.org/10.1111/febs.14699
- Primary Citation of Related Structures:
5ZQV, 5ZT0 - PubMed Abstract:
The rate-limiting enzymes in glycogen metabolism are subject to regulation by reversible phosphorylation. The glycogen-targeted protein phosphatase 1 (PP1) holoenzyme catalyzes their dephosphorylation. It is composed of a catalytic subunit (PP1C) and a glycogen-targeting subunit (G subunit). To date, seven G subunits have been identified. They all contain an RVxF PP1C-binding motif. The interactions between this motif in the skeletal muscle-specific G M and PP1C have been revealed by structural studies. However, whether elements outside of this motif contribute to the interaction with PP1C is not clear. In this study, we found that residues next to the RVxF motif in G M also mediate interactions to PP1C and revealed the mechanism of the interaction by structural studies. Sequence analysis revealed that the PP1C-binding region in G M is highly conserved among G subunits. Consistently, we found that the equivalent region in the liver-enriched G L adopts a similar structure upon binding PP1C. Dephosphorylation experiments indicated that this region and the glycogen-binding region in G M cooperate to stimulate PP1C's activity toward glycogen-associated substrates. DATABASES: The structure factors and coordinates for the PP1Cα-G M (1-99) and PP1Cα-G L (31-105) complexes have been deposited into the Protein Data Bank (http://www.pdb.org), with the accession codes 5ZQV and 5ZT0, respectively.
Organizational Affiliation:
CAS Key Laboratory of Nutrition, Metabolism and Food Safety, Shanghai Institute of Nutrition and Health, Shanghai Institutes for Biological Sciences, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai, China.