Structural polymorphism of the Escherichia coli poly-alpha-L-glutamate synthetase RimK
Arimura, Y., Kono, T., Kino, K., Kurumizaka, H.(2018) Acta Crystallogr F Struct Biol Commun 74: 385-390
- PubMed: 29969101
- DOI: https://doi.org/10.1107/S2053230X18007689
- Primary Citation of Related Structures:
5ZCT - PubMed Abstract:
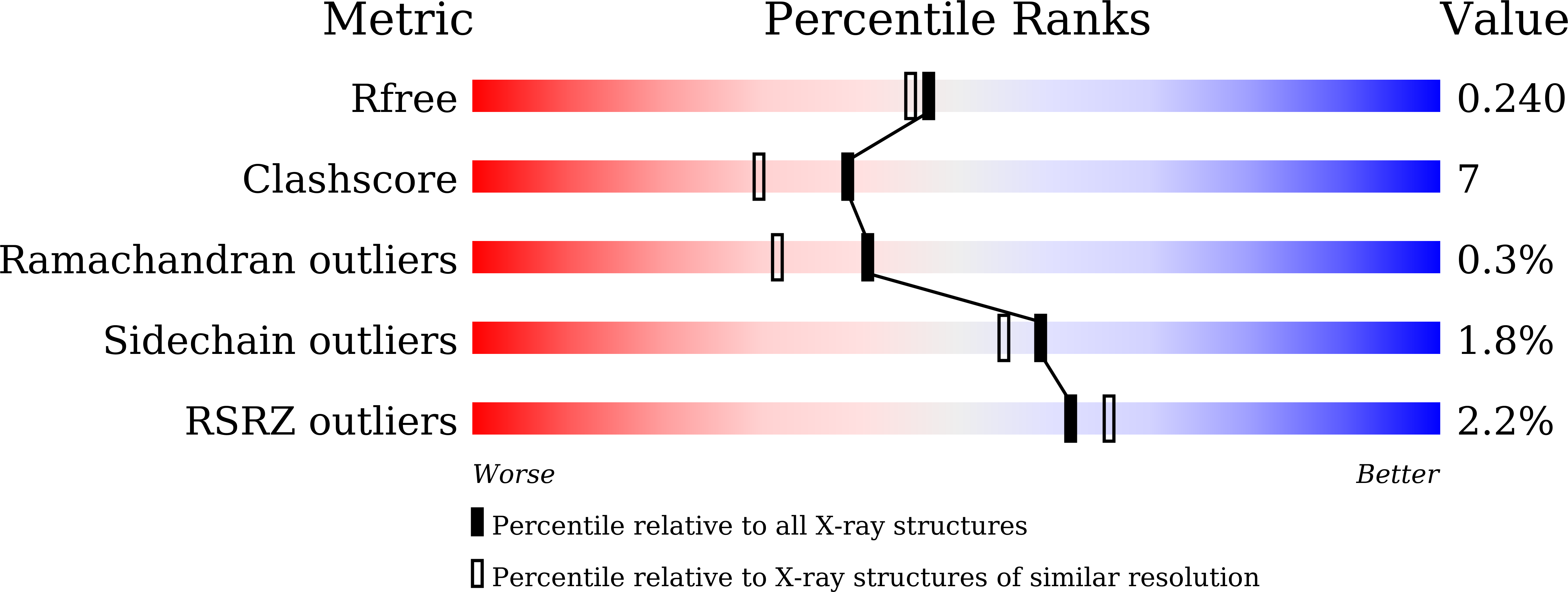
Bacterial RimK is an enzyme that catalyzes the polyglutamylation of the C-terminus of ribosomal protein S6 and the synthesis of poly-α-L-glutamate peptides using L-glutamic acid. In the present study, the crystal structure of the Escherichia coli RimK protein complexed with the ATP analogue AMP-PNP was determined at 2.05 Å resolution. Two different conformations of RimK, closed and open forms, were observed in the crystals. The structural polymorphism revealed in this study provided important information to understand the mechanism by which RimK catalyzes the synthesis of poly-α-L-glutamate peptides and the polyglutamylation of ribosomal protein S6.
Organizational Affiliation:
Laboratory of Structural Biology, Graduate School of Advanced Science and Engineering, Waseda University, 2-2 Wakamatsu-cho, Shinjuku-ku, Tokyo 162-8480, Japan.