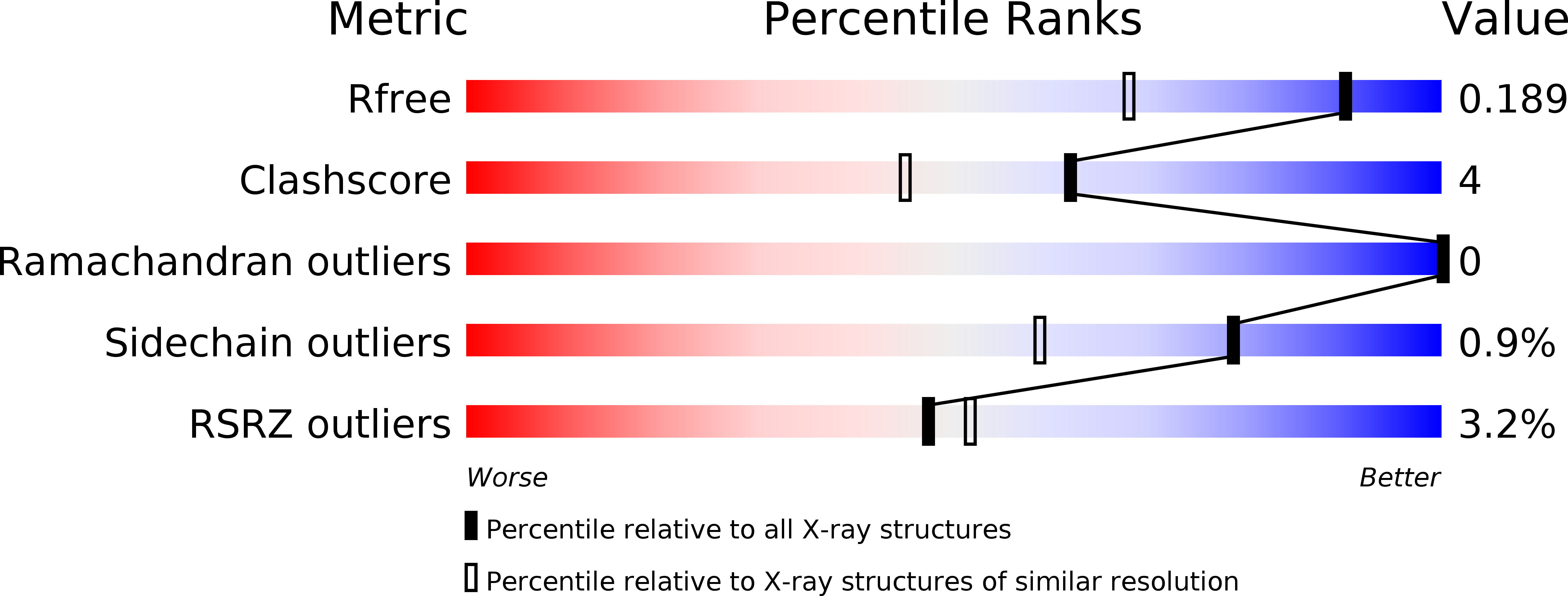
Snapshots of catalysis: Structure of covalently bound substrate trapped in Mycobacterium tuberculosis thiazole synthase (ThiG).
Zhang, J., Zhang, B., Zhao, Y., Yang, X., Huang, M., Cui, P., Zhang, W., Li, J., Zhang, Y.(2018) Biochem Biophys Res Commun 497: 214-219
- PubMed: 29428731
- DOI: https://doi.org/10.1016/j.bbrc.2018.02.056
- Primary Citation of Related Structures:
5Z9Y - PubMed Abstract:
Increasing drug resistance in Mycobacterium tuberculosis (Mtb) has necessitated the design of new anti-mycobacterial drugs with novel targets. Thiazole synthase (ThiG) is an essential enzyme and a potential drug target in Mtb that catalyzes the formation of the thiazole moiety of thiamin-pyrophosphate from 1-deoxy-d-xylulose-5-phosphate (DXP), dehydroglycine and ThiS-thiocarboxylate. To uncover the catalysis mechanism and design potent and selective anti-mycobacterial compounds targeting ThiG, we determined the crystal structure of MtbThiG at 1.5 Å resolution, for the first time, snapshotting a covalently bound substrate trapped in the catalytic pocket. The structure showed a (β/α) 8 barrel overall fold as well as the dimer form of MtbThiG existing in solution. In the central pocket, Lys98 is the key residue forming a protonated carbinolamine intermediate, a functional Schiff base precursor, with DXP. The carbinolamine is further stabilized by active site residues mainly through hydrogen bonds. This work revealed that a protonated carbinolamine is initially formed and then it is dehydrated to the imine form of Schiff base during the early catalysis steps. Our research will provide useful information for understanding the ThiG function and lay the basis for future drug design by targeting this essential protein.
Organizational Affiliation:
Key Laboratory of Medical Molecular Virology, Institute of Medical Microbiology, Department of Infectious Diseases, Huashan Hospital, Fudan University, Shanghai, 200040, China.