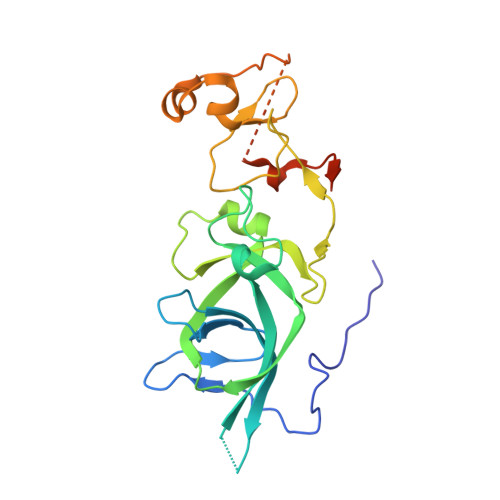
EBS is a bivalent histone reader that regulates floral phase transition in Arabidopsis.
Yang, Z., Qian, S., Scheid, R.N., Lu, L., Chen, X., Liu, R., Du, X., Lv, X., Boersma, M.D., Scalf, M., Smith, L.M., Denu, J.M., Du, J., Zhong, X.(2018) Nat Genet 50: 1247-1253
- PubMed: 30082787
- DOI: https://doi.org/10.1038/s41588-018-0187-8
- Primary Citation of Related Structures:
5Z8L, 5Z8N - PubMed Abstract:
The ability of cells to perceive and translate versatile cues into differential chromatin and transcriptional states is critical for many biological processes 1-5 . In plants, timely transition to a flowering state is crucial for successful reproduction 6-9 . EARLY BOLTING IN SHORT DAY (EBS) is a negative transcriptional regulator that prevents premature flowering in Arabidopsis thaliana 10,11 . We found that EBS contains bivalent bromo-adjacent homology (BAH)-plant homeodomain (PHD) reader modules that bind H3K27me3 and H3K4me3, respectively. We observed co-enrichment of a subset of EBS-associated genes with H3K4me3, H3K27me3, and Polycomb repressor complex 2 (PRC2). Notably, EBS adopted an autoinhibition mode to mediate its switch in binding preference between H3K27me3 and H3K4me3. This binding balance was critical because disruption of either EBS-H3K27me3 or EBS-H3K4me3 interaction induced early floral transition. Our results identify a bivalent chromatin reader capable of recognizing two antagonistic histone marks, and we propose a distinct mechanism of interaction between active and repressive chromatin states.
Organizational Affiliation:
National Key Laboratory of Plant Molecular Genetics, CAS Center for Excellence in Molecular Plant Sciences, Shanghai Center for Plant Stress Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai, China.