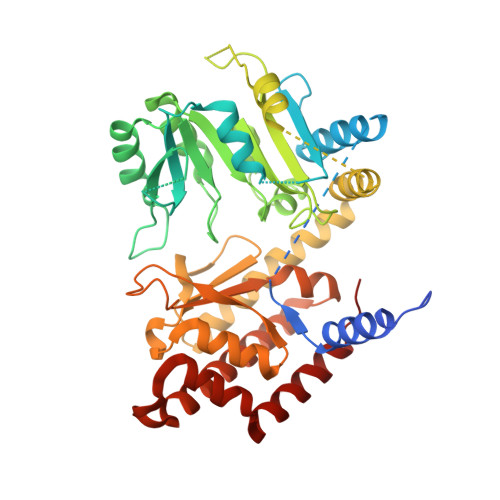
Structure and function of cytoplasmic serine hydroxymethyltransferase from Pichia pastoris
Zhang, M., Wu, W., Chen, Z.(2018) Biochem Biophys Res Commun 496: 753-757
- PubMed: 29339156
- DOI: https://doi.org/10.1016/j.bbrc.2018.01.084
- Primary Citation of Related Structures:
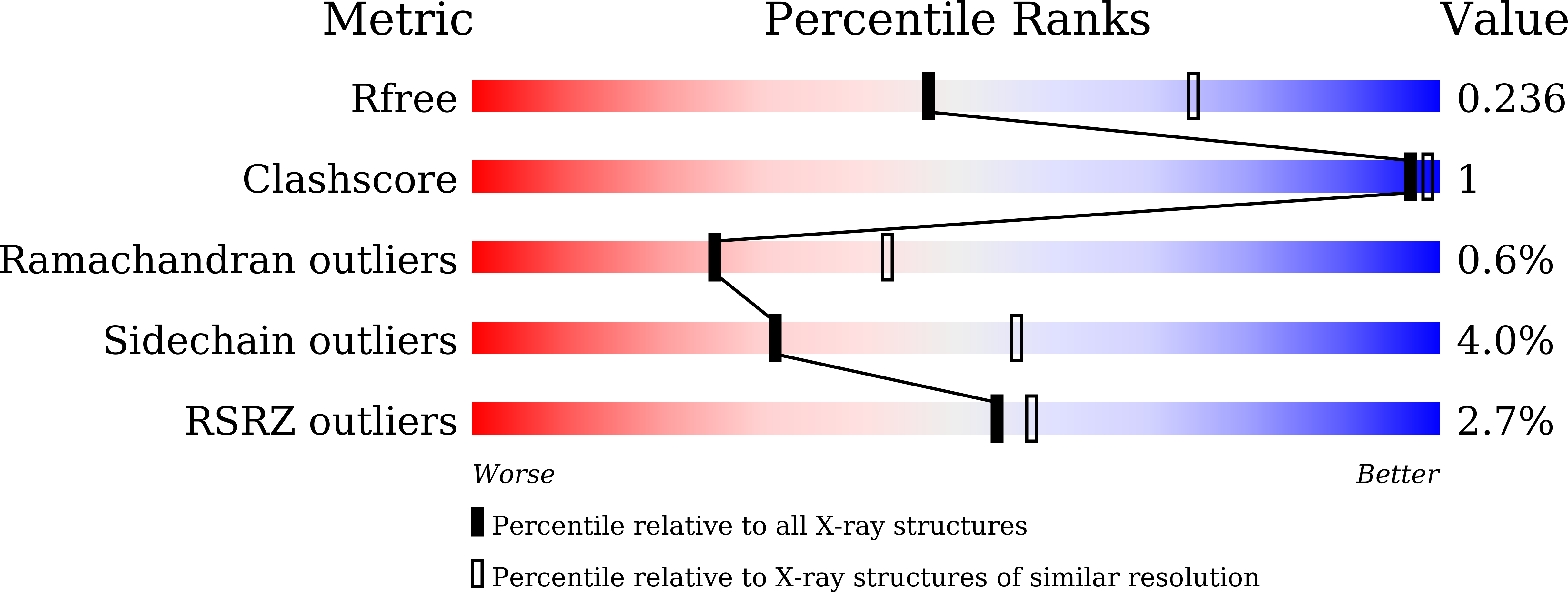
5Z0Y - PubMed Abstract:
Serine hydroxymethyltransferase (SHMT) catalyzes the interconversion of serine and glycine, which is crucial for one carbon metabolism. Here, we report the first crystal structure of cytoplasmic SHMT from Pichia pastoris (pcSHMT) diffracted to 2.5 Å resolution in space group C222 1 . PcSHMT was a contaminant with our target protein expressed in Pichia pastoris and confirmed by mass spectrometry. The overall structure of pcSHMT is similar to Human mitochondrial SHMT and different to E. coli SHMT. Interestingly, the oligomerization of pcSHMT expressed in eukaryotic or prokaryotic system differs significantly and is regulated by pyridoxal-5'-phosphate. Our results revealed a close evolutionary relationship between Pichia pastoris and Human mitochondria.
Organizational Affiliation:
Beijing Advanced Innovation Center for Food Nutrition and Human Health, State Key Laboratory of Agrobiotechnology, China Agricultural University, Beijing 100193, China.