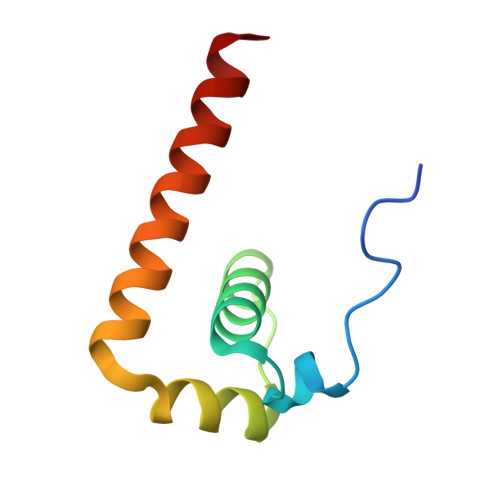
Crystal Structure of the Capsid Protein from Zika Virus.
Shang, Z., Song, H., Shi, Y., Qi, J., Gao, G.F.(2018) J Mol Biol 430: 948-962
- PubMed: 29454707
- DOI: https://doi.org/10.1016/j.jmb.2018.02.006
- Primary Citation of Related Structures:
5YGH - PubMed Abstract:
Recently, Zika virus (ZIKV) emerged as a global public health concern and is distinct from other flaviviruses in many aspects, for example, causing transplacental infection, fetal abnormalities and vector-independent transmission through body fluids in humans. The capsid (C) protein is a multifunctional protein, since it binds to viral RNA in the process of nucleocapsid assembly and plays important roles in virus infection processes by interacting with cellular proteins, modulating cellular metabolism, apoptosis and immune response. Here we solved the crystal structure of ZIKV C protein at a resolution of 1.9Å. The ZIKV C protein structure contains four α helices with a long pre-α1 loop and forms dimers. The unique long pre-α1 loop in ZIKV C contributes to the tighter association of dimeric assembly and renders a divergent hydrophobic feature at the lipid bilayer interface in comparison with the known C structures of West Nile and dengue viruses. We reported the interaction between the ZIKV C protein and lipid droplets through confocal microscopy analysis. Substitutions of key amino acids in the pre-α1 loop of ZIKV C disrupted the interaction with lipid droplets, indicating that the loop is critical for membrane association. We also recognized that ZIKV C protein possesses broad binding capability to different nucleotide types, including single-stranded and double-stranded RNAs or DNAs. Furthermore, the highly positively charged interface, mainly formed by α4 helix, is proposed to be responsible for nucleotide binding. These findings will greatly enhance our understanding of ZIKV C protein, providing information for anti-ZIKV drug design targeting the C protein.
Organizational Affiliation:
Research Network of Immunity and Health (RNIH), Beijing Institutes of Life Science, Chinese Academy of Sciences, Beijing 100101, China; University of Chinese Academy of Sciences, Beijing 100049, China.