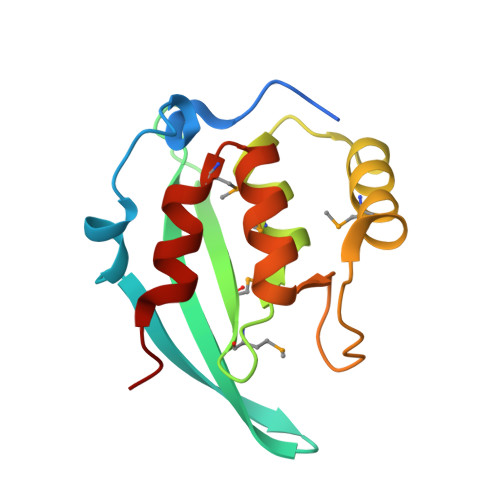
The Inner Nuclear Membrane Protein Bqt4 in Fission Yeast Contains a DNA-Binding Domain Essential for Telomere Association with the Nuclear Envelope.
Hu, C., Inoue, H., Sun, W., Takeshita, Y., Huang, Y., Xu, Y., Kanoh, J., Chen, Y.(2019) Structure 27: 335
- PubMed: 30503780
- DOI: https://doi.org/10.1016/j.str.2018.10.010
- Primary Citation of Related Structures:
5YBX - PubMed Abstract:
Telomeres, the protective caps at the end of the chromosomes, are often associated with the nuclear envelope (NE). Telomere positioning to the NE is dynamically regulated during mitosis and meiosis. One inner nuclear membrane protein, Bqt4, in Schizosaccharomyces pombe plays essential roles in connecting telomeres to the NE. However, the structural basis of Bqt4 in mediating telomere-NE association is not clear. Here, we report the crystal structure of the N-terminal domain of Bqt4. The N-terminal domain of Bqt4 structurally resembles the APSES-family DNA-binding domain and has a moderate double-stranded DNA-binding activity. Disruption of Bqt4-DNA interaction results in telomere detachment from the NE. These data suggest that the DNA-binding activity of Bqt4 may function to prime the chromosome onto the NE and promote telomere-NE association.
Organizational Affiliation:
State Key Laboratory of Molecular Biology, National Center for Protein Science Shanghai, Shanghai Science Research Center, CAS Center for Excellence in Molecular Cell Science, Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences; University of Chinese Academy of Sciences, 333 Haike Road, Shanghai 201210, China.