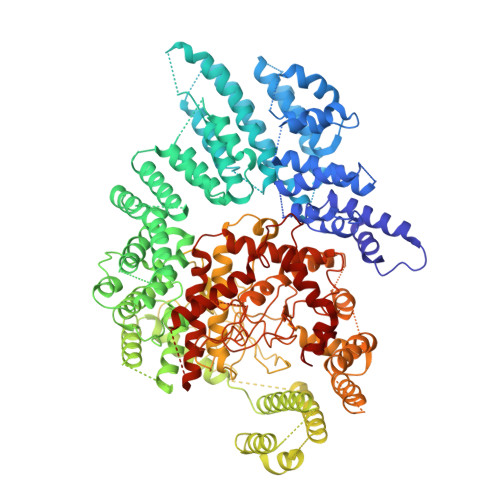
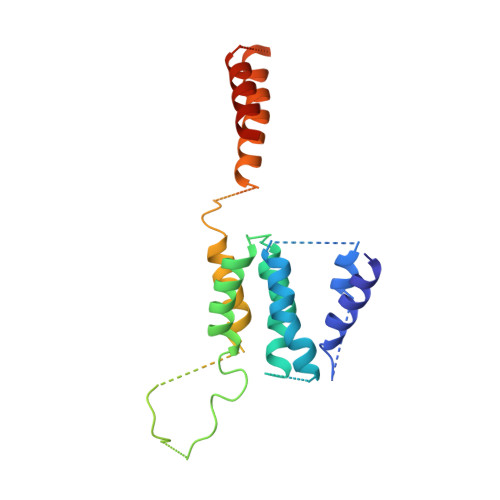
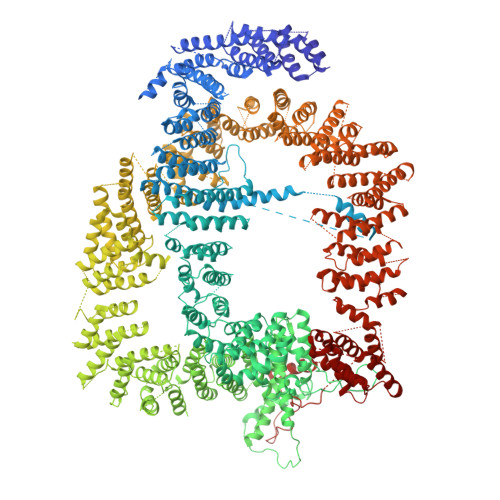
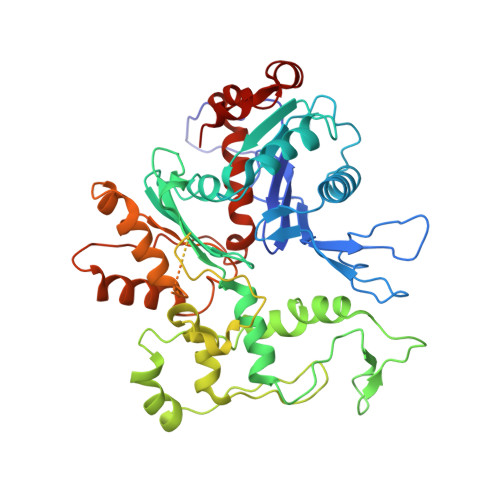
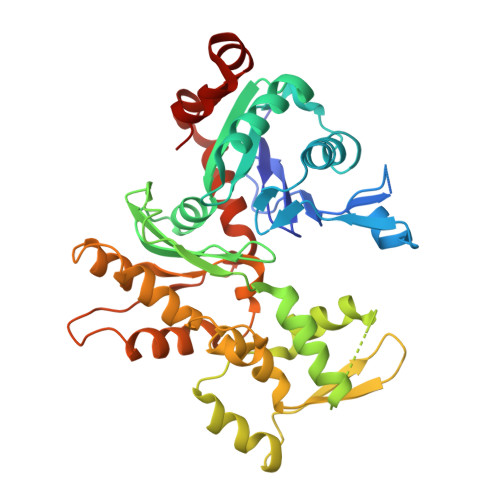
Architecture of the Saccharomyces cerevisiae NuA4/TIP60 complex
Wang, X., Ahmad, S., Zhang, Z., Cote, J., Cai, G.(2018) Nat Commun 9: 1147-1147
- PubMed: 29559617
- DOI: https://doi.org/10.1038/s41467-018-03504-5
- Primary Citation of Related Structures:
5Y81 - PubMed Abstract:
The NuA4/TIP60 acetyltransferase complex is required for gene regulation, DNA repair and cell cycle progression. The limited structural information impeded understanding of NuA4/TIP60 assembly and regulatory mechanism. Here, we report the 4.7 Å cryo-electron microscopy (cryo-EM) structure of a NuA4/TIP60 TEEAA assembly (Tra1, Eaf1, Eaf5, actin and Arp4) and the 7.6 Å cryo-EM structure of a TEEAA-piccolo assembly (Esa1, Epl1, Yng2 and Eaf6). The Tra1 and Eaf1 constitute the assembly scaffold. The Eaf1 SANT domain tightly binds to the LBE and FATC domains of Tra1 by ionic interactions. The actin/Arp4 peripherally associates with Eaf1 HSA domain. The Eaf5/7/3 (TINTIN) and piccolo modules largely pack against the FAT and HEAT repeats of Tra1 and their association depends on Eaf1 N-terminal and HSA regions, respectively. These structures elucidate the detailed architecture and molecular interactions between NuA4 subunits and offer exciting insights into the scaffolding and regulatory mechanisms of Tra1 pseudokinase.
Organizational Affiliation:
Hefei National Laboratory for Physical Sciences at Microscale and School of Life Sciences, University of Science & Technology of China, Hefei, 230026, China. xuejuan@ustc.edu.cn.