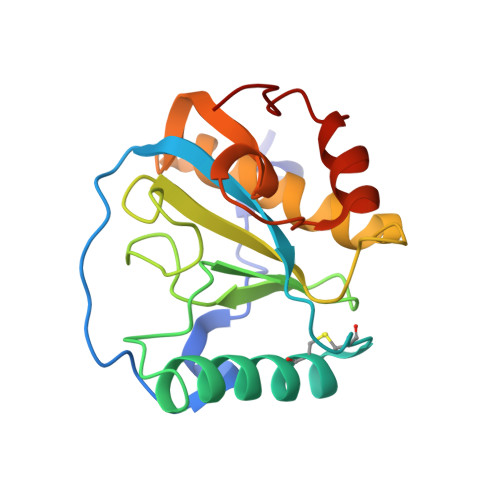
Crystal structure of peptidoglycan recognition protein SA in Apis mellifera (Hymenoptera: Apidae).
Liu, Y., Zhao, X., Naeem, M., An, J.(2018) Protein Sci 27: 893-897
- PubMed: 29396863
- DOI: https://doi.org/10.1002/pro.3383
- Primary Citation of Related Structures:
5XZ3 - PubMed Abstract:
Peptidoglycan recognition protein SA (PGRP-SA) is a key pattern recognition receptor in the insect innate immune system. PGRP-SA can bind to bacterial PGN and activate the Toll pathway, which triggers the expression and release of antimicrobial peptides to prevent bacterial infection. Here, we report the first structure of Apis mellifera PGRP-SA from Hymenoptera at 1.86 Å resolution. The overall architecture of Am-PGRP-SA was similar to the Drosophila PGRP-SA; however, the residues involved in PGN binding groove were not conserved, and the binding pocket was narrower. This structure gives insight into PGN binding characteristics in honeybees.
Organizational Affiliation:
Key Laboratory for Insect-Pollinator Biology of the Ministry of Agriculture, Institute of Apicultural Research, Chinese Academy of Agricultural Sciences, Beijing, 100093, China.