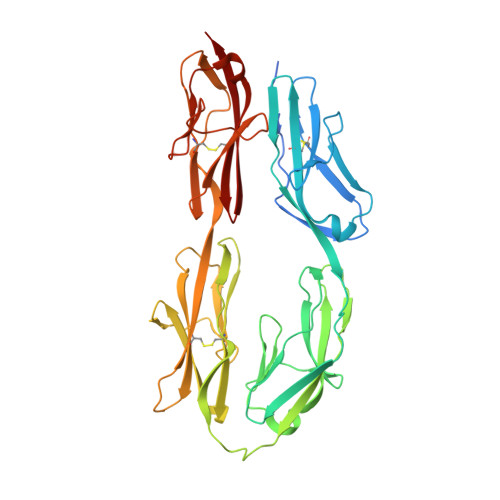
Architecture of cell-cell adhesion mediated by sidekicks.
Tang, H., Chang, H., Dong, Y., Guo, L., Shi, X., Wu, Y., Huang, Y., He, Y.(2018) Proc Natl Acad Sci U S A 115: 9246-9251
- PubMed: 30150416
- DOI: https://doi.org/10.1073/pnas.1801810115
- Primary Citation of Related Structures:
5XWX, 5XX0 - PubMed Abstract:
Cell-cell adhesion is important for cell growth, tissue development, and neural network formation. Structures of cell adhesion molecules have been widely studied by crystallography, revealing the molecular details of adhesion interfaces. However, due to technical limitations, the overall structure and organization of adhesion molecules at cell adhesion interfaces has not been fully investigated. Here, we combine electron microscopy and other biophysical methods to characterize the structure of cell-cell adhesion mediated by the cell adhesion molecule Sidekick (Sidekick-1 and Sidekick-2) and obtain 3D views of the Sidekick-mediated adhesion interfaces as well as the organization of Sidekick molecules between cell membranes by electron tomography. The results suggest that the Ig-like domains and the fibronectin III (FnIII) domains of Sidekicks play different roles in cell adhesion. The Ig-like domains mediate the homophilic transinteractions bridging adjacent cells, while the FnIII domains interact with membranes, resulting in a tight adhesion interface between cells that may contribute to the specificity and plasticity of cell-cell contacts during cell growth and neural development.
Organizational Affiliation:
State Key Laboratory of Molecular Biology, Shanghai Institute of Biochemistry and Cell Biology, CAS Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences 201210 Shanghai, China.