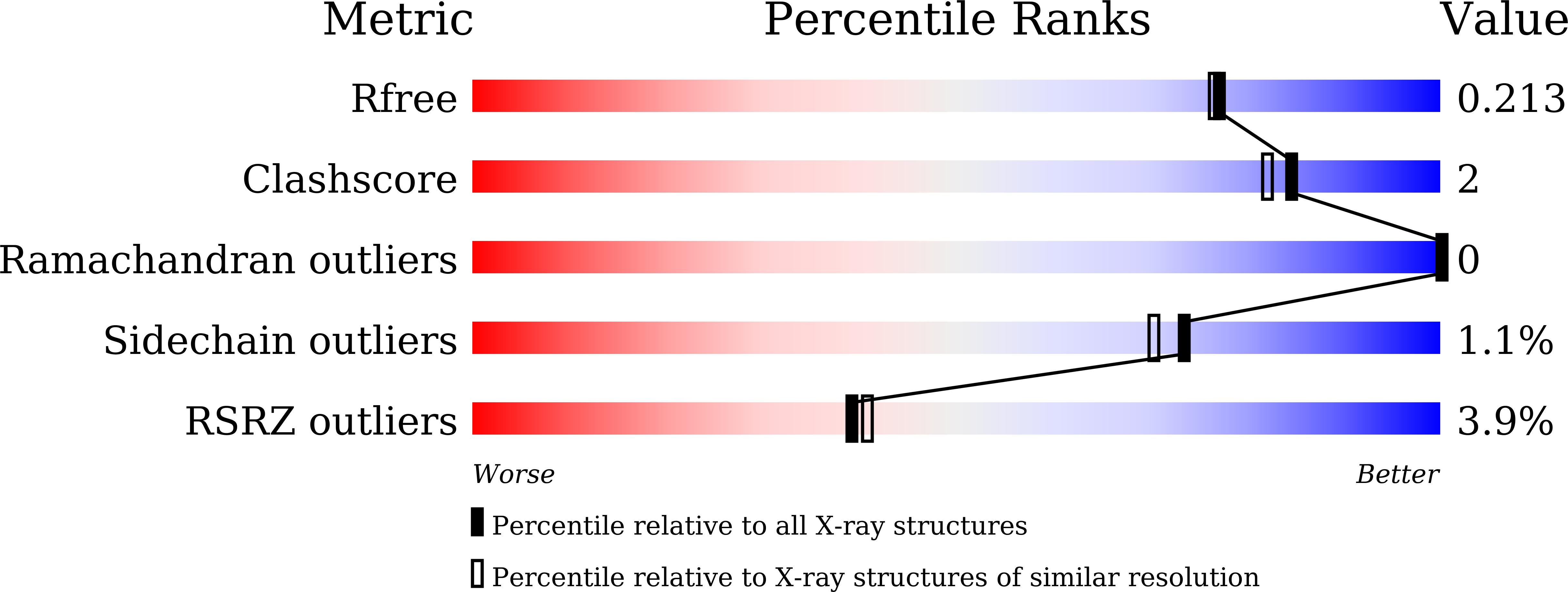
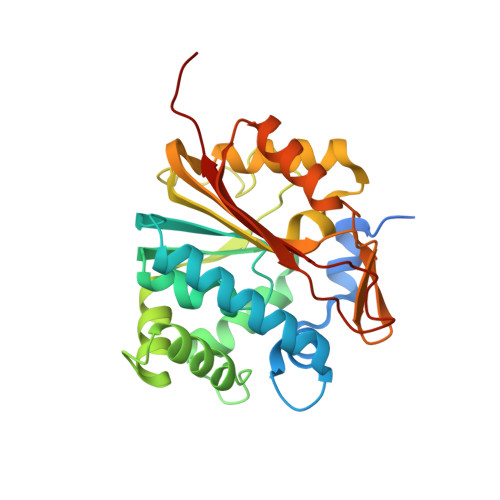
Crystal structures of monkey and mouse nicotinamide N-methyltransferase (NNMT) bound with end product, 1-methyl nicotinamide
Swaminathan, S., Birudukota, S., Thakur, M.K., Parveen, R., Kandan, S., Juluri, S., Shaik, S., Anand, N.N., Burri, R.R., Kristam, R., Hallur, M.S., Rajagopal, S., Schreuder, H., Langer, T., Rudolph, C., Ruf, S., Dhakshinamoorthy, S., Gosu, R., Kannt, A.(2017) Biochem Biophys Res Commun 491: 416-422
- PubMed: 28720493
- DOI: https://doi.org/10.1016/j.bbrc.2017.07.087
- Primary Citation of Related Structures:
5XVK, 5XVQ - PubMed Abstract:
Nicotinamide N-methyltransferase (NNMT) is a S-adenosyl-l-methionine (SAM)-dependent enzyme that catalyzes N-methylation of nicotinamide (NA) and other pyridines to form N-methyl pyridinium ions. Here we report the first ternary complex X-ray crystal structures of monkey NNMT and mouse NNMT in bound form with the primary endogenous product, 1-methyl nicotinamide (MNA) and demethylated cofactor, S-adenosyl-homocysteine (SAH) determined at 2.30 Å and 1.88 Å respectively. The structural fold of these enzymes is identical to human NNMT. It is known that the primary endogenous product catalyzed by NNMT, MNA is a specific inhibitor of NNMT. Our data clearly indicates that the MNA binds to the active site and it would be trapped in the active site due to the formation of the bridge between the pole (long helix, α3) and long C-terminal loop. This might explain the mechanism of MNA acting as a feedback inhibitor of NNMT.
Organizational Affiliation:
Department of Structural Biology, Jubilant Biosys Ltd, Bangalore 560022, India.