The asparagine-rich protein NRP interacts with the Verticillium effector PevD1 and regulates the subcellular localization of cryptochrome 2
Zhou, R., Zhu, T., Han, L., Liu, M., Xu, M., Liu, Y., Han, D., Qiu, D., Gong, Q., Liu, X.(2017) J Exp Bot 68: 3427-3440
- PubMed: 28633330
- DOI: https://doi.org/10.1093/jxb/erx192
- Primary Citation of Related Structures:
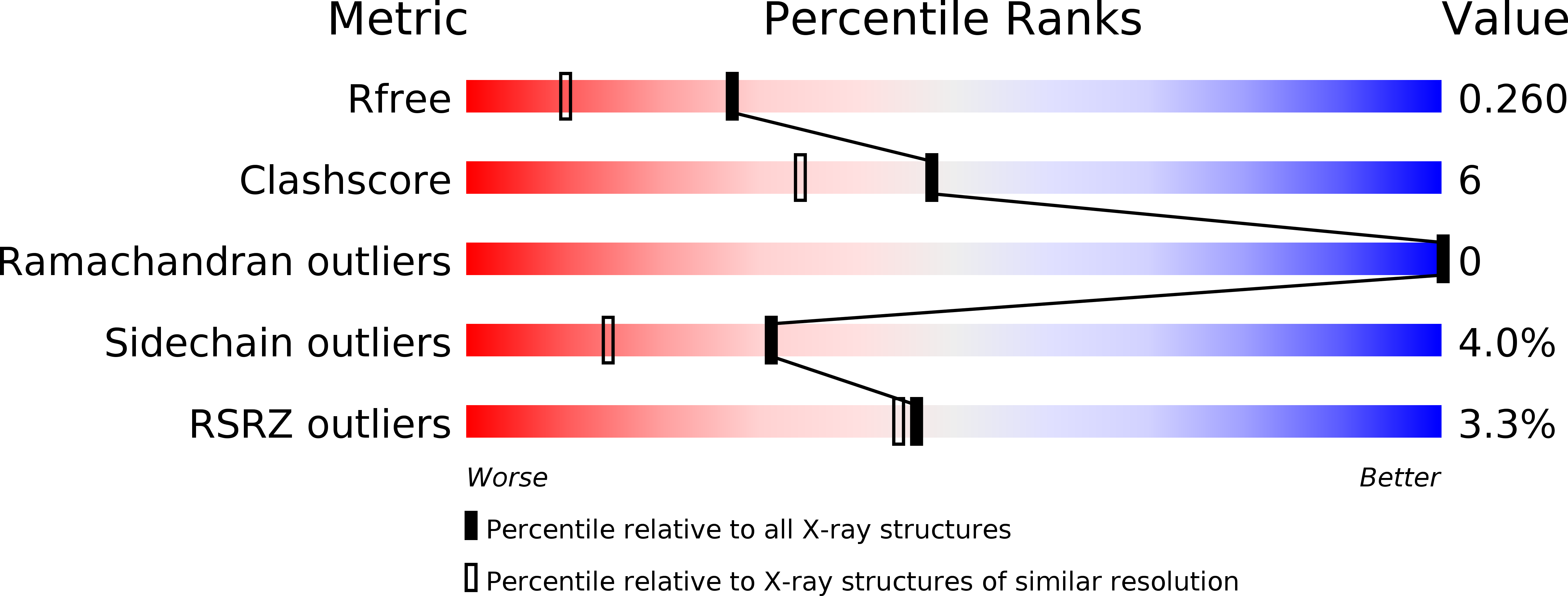
5XMZ - PubMed Abstract:
The soil-borne fungal pathogen Verticillium dahliae infects a wide range of dicotyledonous plants including cotton, tobacco, and Arabidopsis. Among the effector proteins secreted by V. dahliae, the 16 kDa PevD1 induces a hypersensitive response in tobacco. Here we report the high-resolution structure of PevD1 with folds resembling a C2 domain-like structure with a calcium ion bound to the C-terminal acidic pocket. A yeast two-hybrid screen, designed to probe for molecular functions of PevD1, identified Arabidopsis asparagine-rich protein (NRP) as the interacting partner of PevD1. Extending the pathway of V. dahliae effects, which include induction of early flowering in cotton and Arabidopsis, NRP was found to interact with cryptochrome 2 (CRY2), leading to increased cytoplasmic accumulation of CRY2 in a blue light-independent manner. Further physiological and genetic evidence suggests that PevD1 indirectly activates CRY2 by antagonizing NRP functions. The promotion of CRY2-mediated flowering by a fungal effector outlines a novel pathway by which an external stimulus is recognized and transferred in changing a developmental program.
Organizational Affiliation:
State Key Laboratory of Medicinal Chemical Biology, Department of Biochemistry and Molecular Biology, College of Life Sciences, Nankai University, Tianjin 300071, China.