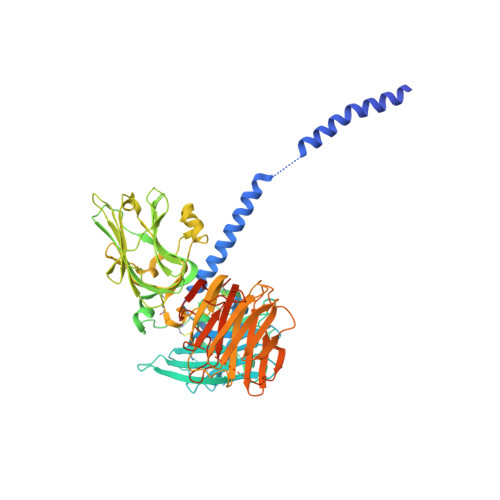
Mechanistic basis for the recognition of laminin-511 by alpha 6 beta 1 integrin.
Takizawa, M., Arimori, T., Taniguchi, Y., Kitago, Y., Yamashita, E., Takagi, J., Sekiguchi, K.(2017) Sci Adv 3: e1701497-e1701497
- PubMed: 28879238
- DOI: https://doi.org/10.1126/sciadv.1701497
- Primary Citation of Related Structures:
5XAU - PubMed Abstract:
Laminins regulate diverse cellular functions through interaction with integrins. Two regions of laminins-three laminin globular domains of the α chain (LG1-3) and the carboxyl-terminal tail of the γ chain (γ-tail)-are required for integrin binding, but it remains unclear how the γ-tail contributes to the binding. We determined the crystal structure of the integrin binding fragment of laminin-511, showing that the γ-tail extends to the bottom face of LG1-3. Electron microscopic imaging combined with biochemical analyses showed that integrin binds to the bottom face of LG1-3 with the γ1-tail apposed to the metal ion-dependent adhesion site (MIDAS) of integrin β1. These findings are consistent with a model in which the γ-tail coordinates the metal ion in the MIDAS through its Glu residue.
Organizational Affiliation:
Division of Matrixome Research and Application, Institute for Protein Research, Osaka University, 3-2 Yamadaoka, Suita, Osaka 565-0871, Japan.