Replication regulation protein
Kim, J., Cho, Y.To be published.
Experimental Data Snapshot
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| DNA polymerase III subunit beta | 386 | Escherichia coli O157:H7 | Mutation(s): 0 Gene Names: dnaN, Z5192, ECs4636 EC: 2.7.7.7 | 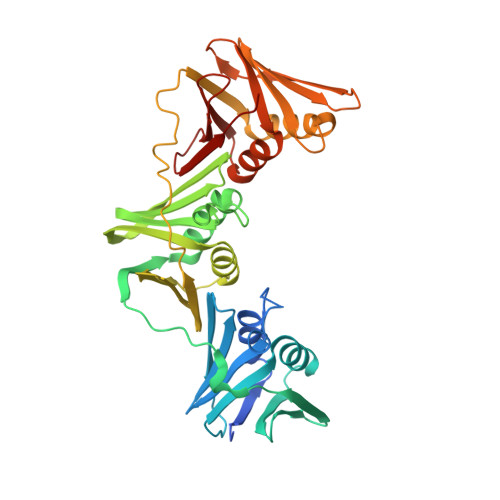 | |
UniProt | |||||
Find proteins for P0A988 (Escherichia coli (strain K12)) Explore P0A988 Go to UniProtKB: P0A988 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P0A988 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| DnaA regulatory inactivator Hda | 253 | Escherichia coli O157:H7 | Mutation(s): 0 Gene Names: hda, Z3759, ECs3358 | 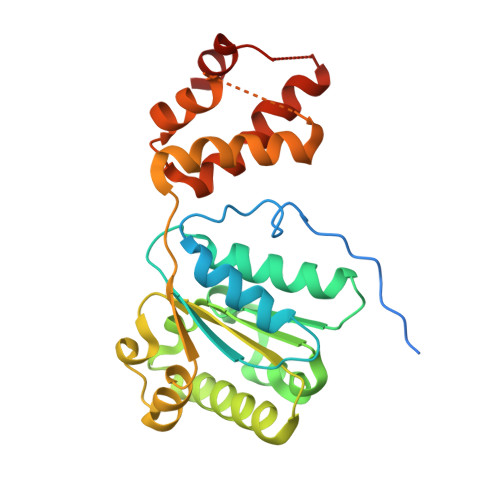 | |
UniProt | |||||
Find proteins for P69931 (Escherichia coli (strain K12)) Explore P69931 Go to UniProtKB: P69931 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P69931 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| ADP Query on ADP | K [auth E], N [auth F], P [auth G], S [auth H] | ADENOSINE-5'-DIPHOSPHATE C10 H15 N5 O10 P2 XTWYTFMLZFPYCI-KQYNXXCUSA-N |  | ||
| GOL Query on GOL | I [auth C], J [auth D], M [auth E], R [auth G] | GLYCEROL C3 H8 O3 PEDCQBHIVMGVHV-UHFFFAOYSA-N |  | ||
| MG Query on MG | L [auth E], O [auth F], Q [auth G], T [auth H] | MAGNESIUM ION Mg JLVVSXFLKOJNIY-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 102.112 | α = 90 |
| b = 149.542 | β = 90 |
| c = 200.78 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |
| PHENIX | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Research Foundation of Korea funded by the Korea Government | Korea, Republic Of | NRF-2015R1A2A1A05001694 |
| National Research Foundation of Korea funded by the Korea Government | Korea, Republic Of | 2012054226 |
| National Research Foundation of Korea funded by the Korea Government | Korea, Republic Of | 20120008833 |