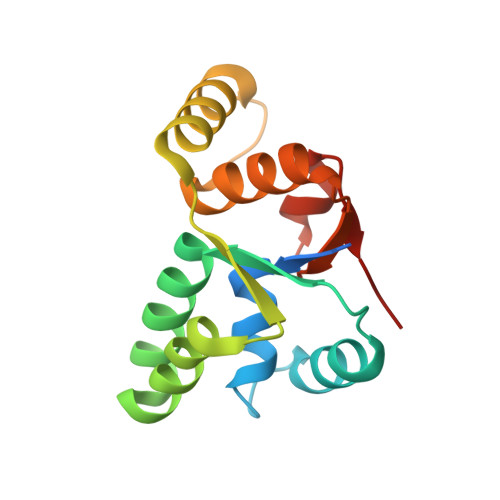
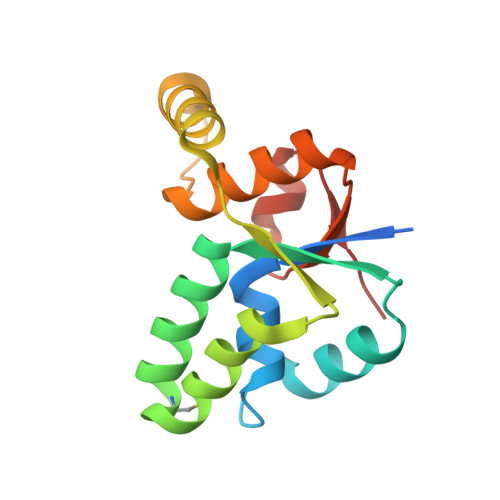
Crystal structure of Mycobacterium tuberculosis VapC20 toxin and its interactions with cognate antitoxin, VapB20, suggest a model for toxin-antitoxin assembly.
Deep, A., Kaundal, S., Agarwal, S., Singh, R., Thakur, K.G.(2017) FEBS J 284: 4066-4082
- PubMed: 28986943
- DOI: https://doi.org/10.1111/febs.14289
- Primary Citation of Related Structures:
5WZ4, 5WZF - PubMed Abstract:
VapBCs, virulence-associated proteins, are the most abundant type II toxin-antitoxin (TA) systems in prokaryotes. Under normal conditions, toxin and antitoxin interact to form a heterooctameric complex, which upon binding to operator sites, inhibits their own expression. Under stress conditions, the VapB antitoxin is degraded by cellular proteases to release a free VapC toxin, which in turn inhibits cell growth mainly by targeting protein translation. However, the intermediate steps involved in the assembly of the heterooctameric complex have not been resolved. Here, we report a 1.75 Å resolution crystal structure of VapC20, a Sarcin-Ricin loop cleaving toxin from type II TA system of Mycobacterium tuberculosis. Using analytical ultracentrifugation (AUC) studies, we show that VapC20 exists as a homodimer in solution. The structural analysis of VapC homologs further suggests that VapCs form homodimers. We demonstrate that VapC20 is an obligate homodimer, and its self-association is critical for its folding and activity. Surface plasmon resonance experiments suggest that VapC20 interacts with its cognate antitoxin VapB20 to form a stable complex with nanomolar affinity. A high association rate coupled with a very slow dissociation rate ensures minimal toxicity under normal growth conditions. AUC studies reveal that VapB20 also exists as a homodimer in solution and further associates with VapC20 dimers to form heterotetramers and heterooctamers in a concentration-dependent manner. The results presented here provide valuable insights into the assembly of VapBC family of toxins which is essential for their function and regulation.
Organizational Affiliation:
Structural Biology Laboratory, G. N. Ramachandran Protein Centre, Council of Scientific and Industrial Research-Institute of Microbial Technology (CSIR-IMTECH), Chandigarh, India.