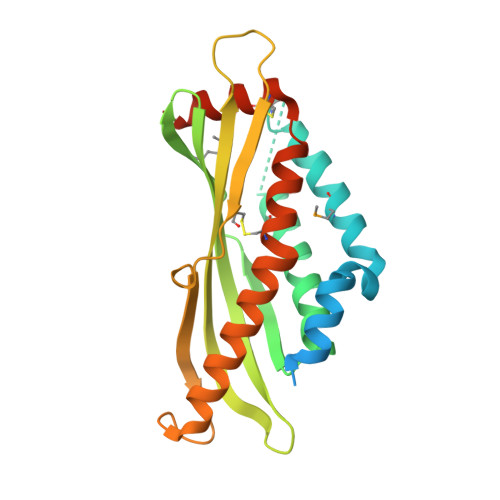
The three dimensional structure of Bovine Salivary Protein 30b (BSP30b) and its interaction with specific rumen bacteria.
Zhang, H., Burrows, J., Card, G.L., Attwood, G., Wheeler, T.T., Arcus, V.L.(2019) PLoS One 14: e0206709-e0206709
- PubMed: 30978191
- DOI: https://doi.org/10.1371/journal.pone.0206709
- Primary Citation of Related Structures:
5WD6, 6O1T - PubMed Abstract:
Bovine Salivary Protein 30b (BSP30b) is a member of the tubular lipid-binding (TULIP) superfamily that includes the human bactericidal/permeability-increasing proteins (BPI), lipopolysaccharide binding proteins (LBP) and palate, lung, and nasal epithelium carcinoma-associated proteins (PLUNC). BSP30b is most closely related to the PLUNC family and is predominantly found in bovine saliva. There are four BSP30 isoforms (BSP30a-d) and collectively, they are the most abundant protein component of bovine saliva. The PLUNC family members are proposed to be lipid binding proteins, although in most cases their lipid ligands are unknown. Here, we present the X-ray crystal structure of BSP30b at 2.0 Å resolution. We used a double methionine mutant and Se-Met SAD phasing to solve the structure. The structure adopts a curved cylindrical form with a hydrophobic channel formed by an α/β wrap, which is consistent with the TULIP superfamily. The structure of BSP30b in complex with oleic acid is also presented where the ligand is accommodated within the hydrophobic channel. The electron density for oleic acid suggests that the ligand is only partially occupied in the binding site implying that oleic acid may not be the preferred ligand. GFP-tagged BSP30b binds to the surface of olive oil droplets, as observed under fluorescent microscopy, and acts as a surfactant consistent with its association with decreased susceptibility to bloat in cattle. Bacteria extracted directly from bovine rumen contents indicate that the GFP_BSP30b fusion protein binds to a small number of selected bacterial species in vivo. These results suggest that BSP30b may bind to bacterial lipids from specific species and that this abundant protein may have important biological roles via interacting with rumen bacteria during feeding and rumination.
Organizational Affiliation:
School of Science, University of Waikato, Hamilton, New Zealand.