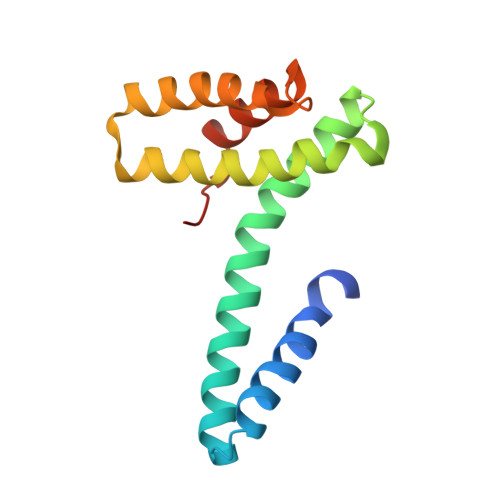
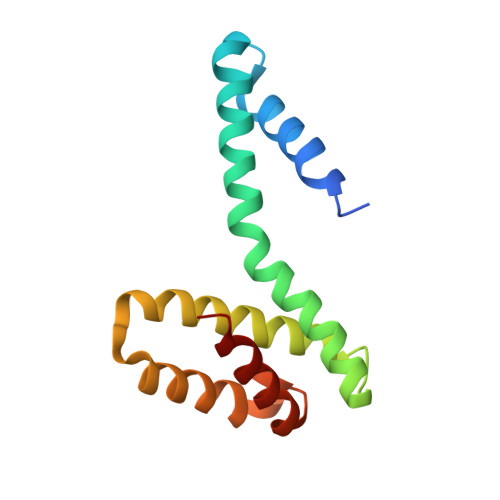
Model for a novel membrane envelope in a filamentous hyperthermophilic virus.
Egelman, E.H., Kasson, P.M., DiMaio, F., Yu, X., Lucas-Staat, S., Krupovic, M., Schouten, S., Prangishvili, D.(2017) Elife 6
- PubMed: 28639939
- DOI: https://doi.org/10.7554/eLife.26268
- Primary Citation of Related Structures:
5W7G - PubMed Abstract:
Biological membranes create compartments, and are usually formed by lipid bilayers. However, in hyperthermophilic archaea that live optimally at temperatures above 80°C the membranes are monolayers which resemble fused bilayers. Many double-stranded DNA viruses which parasitize such hosts, including the filamentous virus AFV1 of Acidianus hospitalis , are enveloped with a lipid-containing membrane. Using cryo-EM, we show that the membrane in AFV1 is a ~2 nm-thick monolayer, approximately half the expected membrane thickness, formed by host membrane-derived lipids which adopt a U-shaped 'horseshoe' conformation. We hypothesize that this unusual viral envelope structure results from the extreme curvature of the viral capsid, as 'horseshoe' lipid conformations favor such curvature and host membrane lipids that permit horseshoe conformations are selectively recruited into the viral envelope. The unusual envelope found in AFV1 also has many implications for biotechnology, since this membrane can survive the most aggressive conditions involving extremes of temperature and pH.
Organizational Affiliation:
Department of Molecular Physiology and Biological Physics, University of Virginia, Charlottesville, United States.