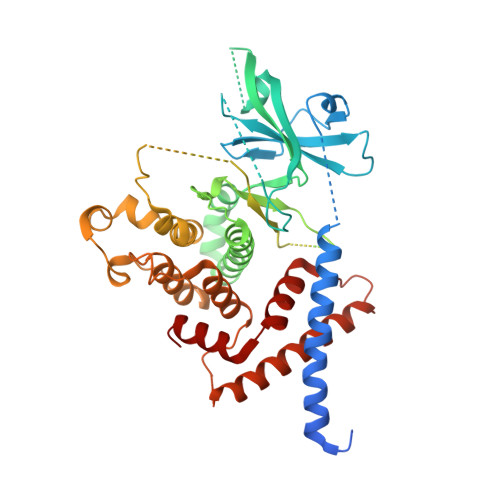
Structure of SgK223 pseudokinase reveals novel mechanisms of homotypic and heterotypic association.
Patel, O., Griffin, M.D.W., Panjikar, S., Dai, W., Ma, X., Chan, H., Zheng, C., Kropp, A., Murphy, J.M., Daly, R.J., Lucet, I.S.(2017) Nat Commun 8: 1157-1157
- PubMed: 29079850
- DOI: https://doi.org/10.1038/s41467-017-01279-9
- Primary Citation of Related Structures:
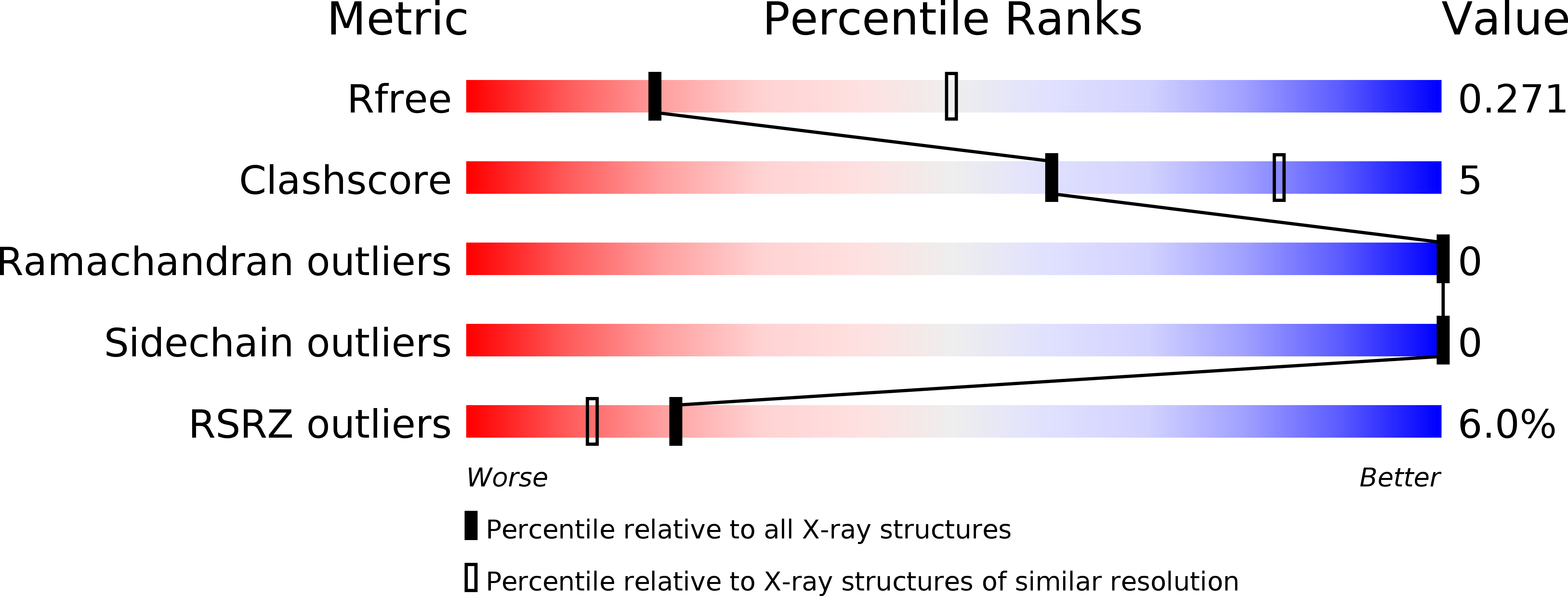
5VE6 - PubMed Abstract:
The mammalian pseudokinase SgK223, and its structurally related homologue SgK269, are oncogenic scaffolds that nucleate the assembly of specific signalling complexes and regulate tyrosine kinase signalling. Both SgK223 and SgK269 form homo- and hetero-oligomers, a mechanism that underpins a diversity of signalling outputs. However, mechanistic insights into SgK223 and SgK269 homo- and heterotypic association are lacking. Here we present the crystal structure of SgK223 pseudokinase domain and its adjacent N- and C-terminal helices. The structure reveals how the N- and C-regulatory helices engage in a novel fold to mediate the assembly of a high-affinity dimer. In addition, we identified regulatory interfaces on the pseudokinase domain required for the self-assembly of large open-ended oligomers. This study highlights the diversity in how the kinase fold mediates non-catalytic functions and provides mechanistic insights into how the assembly of these two oncogenic scaffolds is achieved in order to regulate signalling output.
Organizational Affiliation:
The Walter and Eliza Hall Institute of Medical Research, Parkville, VIC, 3052, Australia. patel.o@wehi.edu.au.