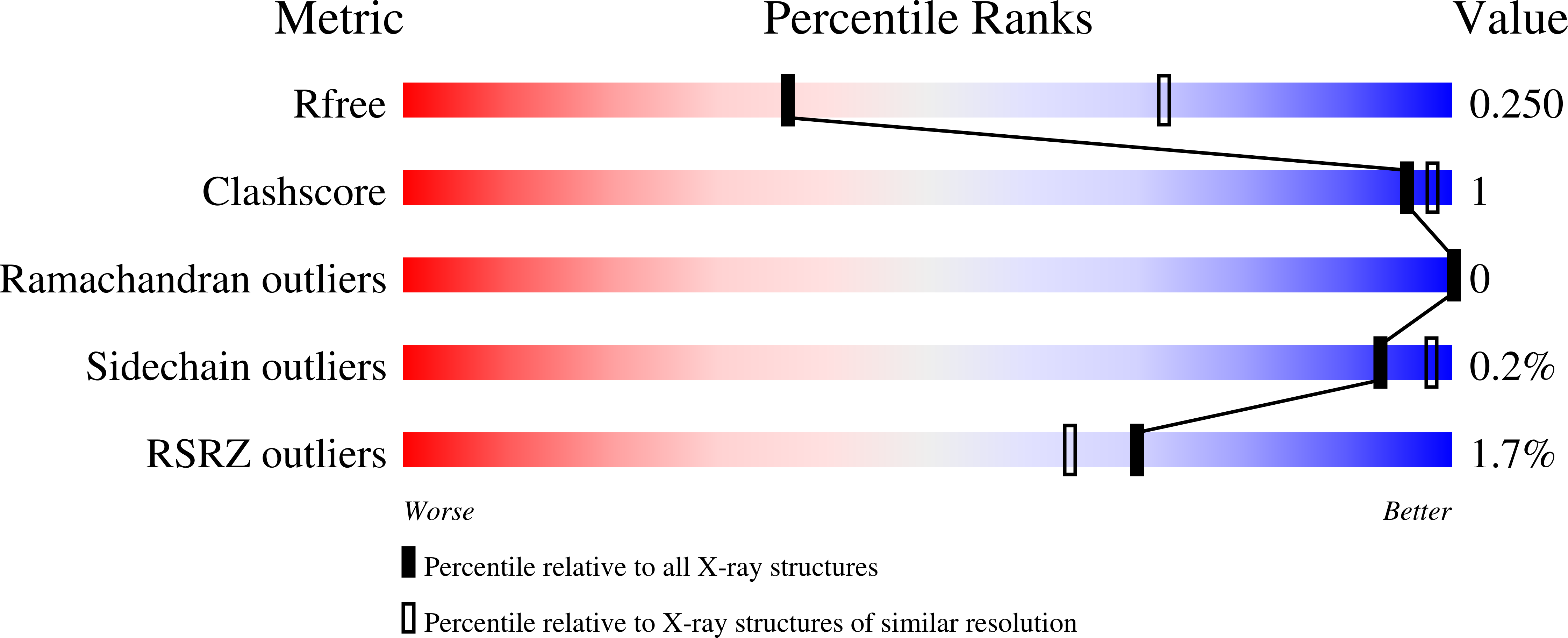
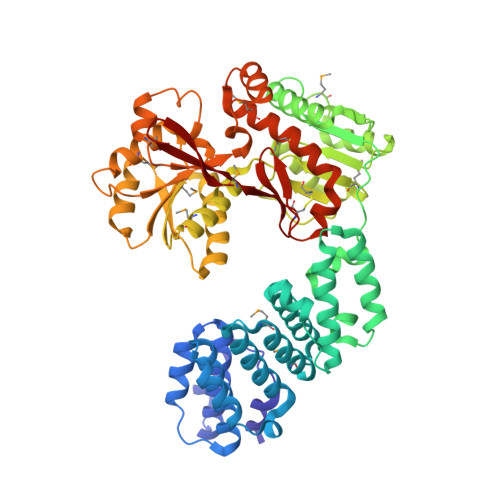
Structural analyses of the Haemophilus influenzae peptidoglycan synthase activator LpoA suggest multiple conformations in solution.
Sathiyamoorthy, K., Vijayalakshmi, J., Tirupati, B., Fan, L., Saper, M.A.(2017) J Biol Chem 292: 17626-17642
- PubMed: 28887305
- DOI: https://doi.org/10.1074/jbc.M117.804997
- Primary Citation of Related Structures:
4P29, 5KCN, 5VAT, 5VBG - PubMed Abstract:
In many Gram-negative bacteria, the peptidoglycan synthase PBP1A requires the outer membrane lipoprotein LpoA for constructing a functional peptidoglycan required for bacterial viability. Previously, we have shown that the C-terminal domain of Haemophilus influenzae LpoA ( Hi LpoA) has a highly conserved, putative substrate-binding cleft between two α/β lobes. Here, we report a 2.0 Å resolution crystal structure of the Hi LpoA N-terminal domain. Two subdomains contain tetratricopeptide-like motifs that form a concave groove, but their relative orientation differs by ∼45° from that observed in an NMR structure of the Escherichia coli LpoA N domain. We also determined three 2.0-2.8 Å resolution crystal structures containing four independent full-length Hi LpoA molecules. In contrast to an elongated model previously suggested for E. coli LpoA, each Hi LpoA formed a U-shaped structure with a different C-domain orientation. This resulted from both N-domain twisting and rotation of the C domain (up to 30°) at the end of the relatively immobile interdomain linker. Moreover, a previously predicted hinge between the lobes of the LpoA C domain exhibited variations of up to 12°. Small-angle X-ray scattering data revealed excellent agreement with a model calculated by normal mode analysis from one of the full-length Hi LpoA molecules but even better agreement with an ensemble of this molecule and two of the partially extended normal mode analysis-predicted models. The different LpoA structures helped explain how an outer membrane-anchored LpoA can either withdraw from or extend toward the inner membrane-bound PBP1A through peptidoglycan gaps and hence regulate the synthesis of peptidoglycan necessary for bacterial viability.
Organizational Affiliation:
From the Program in Biophysics and.