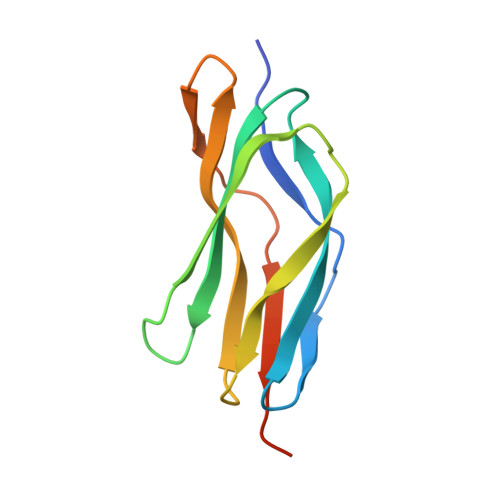
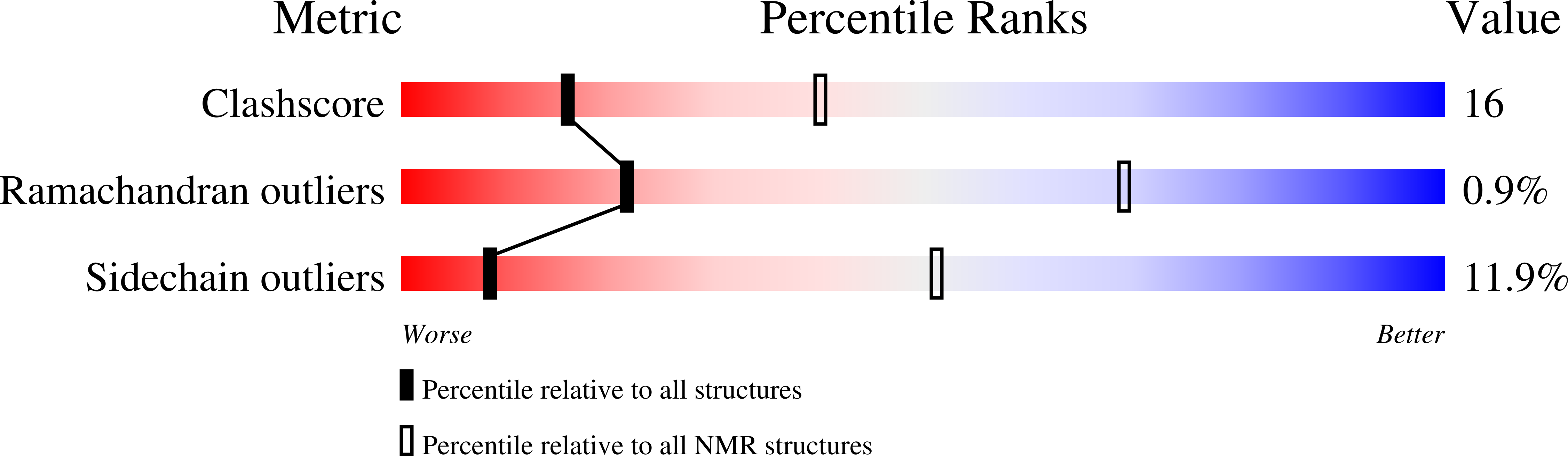Molecular Architecture of the Major Membrane Ring Component of the Nuclear Pore Complex.
Upla, P., Kim, S.J., Sampathkumar, P., Dutta, K., Cahill, S.M., Chemmama, I.E., Williams, R., Bonanno, J.B., Rice, W.J., Stokes, D.L., Cowburn, D., Almo, S.C., Sali, A., Rout, M.P., Fernandez-Martinez, J.(2017) Structure 25: 434-445
- PubMed: 28162953
- DOI: https://doi.org/10.1016/j.str.2017.01.006
- Primary Citation of Related Structures:
5TVZ - PubMed Abstract:
The membrane ring that equatorially circumscribes the nuclear pore complex (NPC) in the perinuclear lumen of the nuclear envelope is composed largely of Pom152 in yeast and its ortholog Nup210 (or Gp210) in vertebrates. Here, we have used a combination of negative-stain electron microscopy, nuclear magnetic resonance, and small-angle X-ray scattering methods to determine an integrative structure of the ∼120 kDa luminal domain of Pom152. Our structural analysis reveals that the luminal domain is formed by a flexible string-of-pearls arrangement of nine repetitive cadherin-like Ig-like domains, indicating an evolutionary connection between NPCs and the cell adhesion machinery. The 16 copies of Pom152 known to be present in the yeast NPC are long enough to form the observed membrane ring, suggesting how interactions between Pom152 molecules help establish and maintain the NPC architecture.
Organizational Affiliation:
Skirball Institute and Department of Cell Biology, New York University School of Medicine, New York, NY 10016, USA; Laboratory of Cellular and Structural Biology, The Rockefeller University, Box 213, 1230 York Avenue, New York, NY 10065, USA.