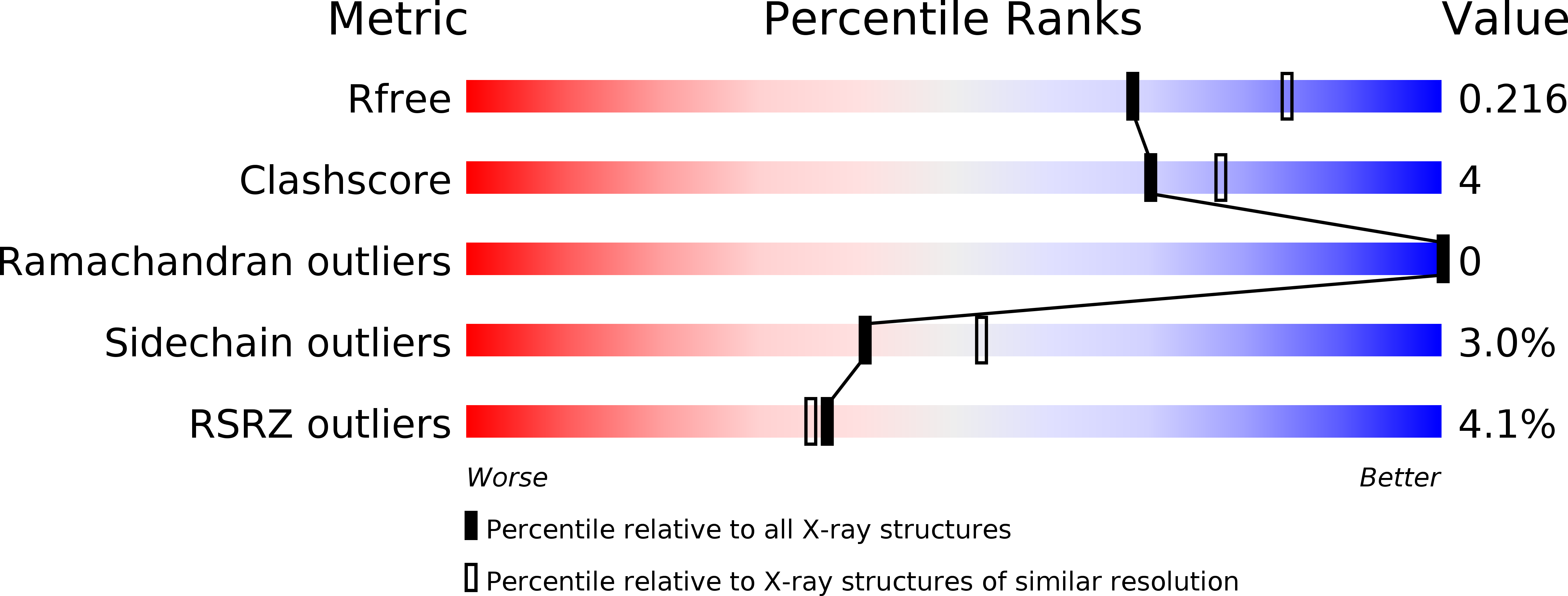
Crystal Structure of Os79 (Os04g0206600) from Oryza sativa: A UDP-glucosyltransferase Involved in the Detoxification of Deoxynivalenol.
Wetterhorn, K.M., Newmister, S.A., Caniza, R.K., Busman, M., McCormick, S.P., Berthiller, F., Adam, G., Rayment, I.(2016) Biochemistry 55: 6175-6186
- PubMed: 27715009
- DOI: https://doi.org/10.1021/acs.biochem.6b00709
- Primary Citation of Related Structures:
5TMB, 5TMD, 5TME - PubMed Abstract:
Fusarium head blight is a plant disease with significant agricultural and health impact which affects cereal crops such as wheat, barley, and maize and is characterized by reduced grain yield and the accumulation of trichothecene mycotoxins such as deoxynivalenol (DON). Studies have identified trichothecene production as a virulence factor in Fusarium graminearum and have linked DON resistance to the ability to form DON-3-O-glucoside in wheat. Here, the structures of a deoxynivalenol:UDP-glucosyltransferase (Os79) from Oryza sativa are reported in complex with UDP in an open conformation, in complex with UDP in a closed conformation, and in complex with UDP-2-fluoro-2-deoxy-d-glucose and trichothecene at 1.8, 2.3, and 2.2 Å resolution, respectively. The active site of Os79 lies in a groove between the N-terminal acceptor and the C-terminal donor-binding domains. Structural alignments reveal that Os79 likely utilizes a catalytic mechanism similar to those of other plant UGTs, with His 27 activating the trichothecene O3 hydroxyl for nucleophilic attack at C1' of the UDP-glucose donor. Kinetic analysis of mutant Os79 revealed that Thr 291 plays a critical role in catalysis as a catalytic acid or to position the UDP moiety during the nucleophilic attack. Steady-state kinetic analysis demonstrated that Os79 conjugates multiple trichothecene substrates such as DON, nivalenol, isotrichodermol, and HT-2 toxin, but not T-2 toxin. These data establish a foundation for understanding substrate specificity and activity in this enzyme and can be used to guide future efforts to increase DON resistance in cereal crops.
Organizational Affiliation:
Department of Biochemistry, University of Wisconsin-Madison , Madison, Wisconsin 53706, United States.