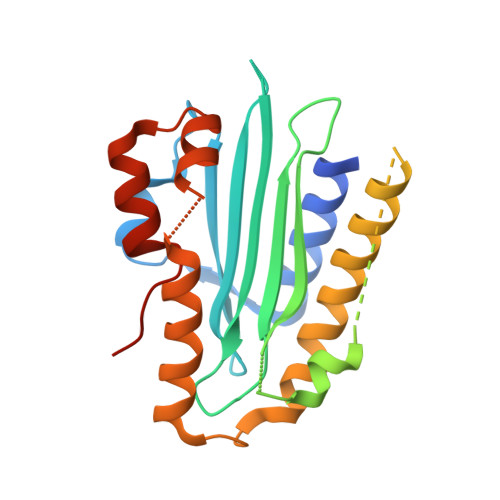
Evolution and molecular mechanism of four-electron reducing ferredoxin-dependent bilin reductases from oceanic phages.
Ledermann, B., Schwan, M., Sommerkamp, J.A., Hofmann, E., Beja, O., Frankenberg-Dinkel, N.(2018) FEBS J 285: 339-356
- PubMed: 29156487
- DOI: https://doi.org/10.1111/febs.14341
- Primary Citation of Related Structures:
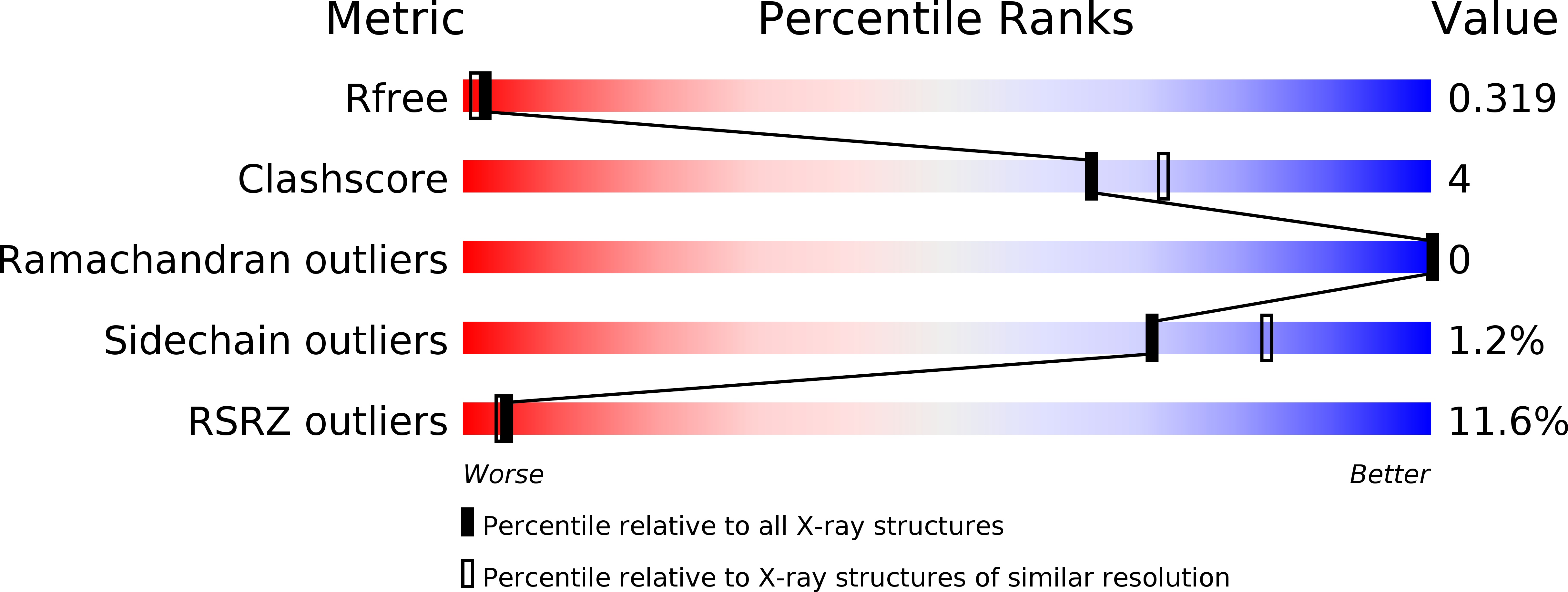
5OWG - PubMed Abstract:
Ferredoxin-dependent bilin reductases (FDBRs) are a class of enzymes reducing the heme metabolite biliverdin IXα (BV) to form open-chain tetrapyrroles used for light-perception and light-harvesting in photosynthetic organisms. Thus far, seven FDBR families have been identified, each catalysing a distinct reaction and either transferring two or four electrons from ferredoxin onto the substrate. The newest addition to the family is PcyX, originally identified from metagenomics data derived from phage. Phylogenetically, PcyA is the closest relative catalysing the reduction of BV to phycocyanobilin. PcyX, however, converts the same substrate to phycoerythrobilin, resembling the reaction catalysed by cyanophage PebS. Within this study, we aimed at understanding the evolution of catalytic activities within FDBRs using PcyX as an example. Additional members of the PcyX clade and a remote member of the PcyA family were investigated to gain insights into catalysis. Biochemical data in combination with the PcyX crystal structure revealed that a conserved aspartate-histidine pair is critical for activity. Interestingly, the same residues are part of a catalytic Asp-His-Glu triad in PcyA, including an additional Glu. While this Glu residue is replaced by Asp in PcyX, it is not involved in catalysis. Substitution back to a Glu failed to convert PcyX to a PcyA. Therefore, the change in regiospecificity is not only caused by individual catalytic amino acid residues. Rather the combination of the architecture of the active site with the positioning of the substrate triggers specific proton transfer yielding the individual phycobilin products.
Organizational Affiliation:
Department of Biology, Microbiology, Technical University Kaiserslautern, Germany.