5O6V
The cryo-EM structure of Tick-borne encephalitis virus complexed with Fab fragment of neutralizing antibody 19/1786
- PDB DOI: https://doi.org/10.2210/pdb5O6V/pdb
- EM Map EMD-3754: EMDB EMDataResource
- Classification: VIRUS
- Organism(s): Tick-borne encephalitis virus (strain HYPR), Mus musculus
- Mutation(s): No
- Deposited: 2017-06-07 Released: 2018-02-07
- Funding Organization(s): European Research Council, European Molecular Biology Organization, Czech Science Foundation
Experimental Data Snapshot
- Method: ELECTRON MICROSCOPY
- Resolution: 3.90 Å
- Aggregation State: PARTICLE
- Reconstruction Method: SINGLE PARTICLE
wwPDB Validation 3D Report Full Report
This is version 1.5 of the entry. See complete history.
Macromolecules
Find similar proteins by:
(by identity cutoff) | 3D Structure
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Envelope protein | 496 | Tick-borne encephalitis virus (strain HYPR) | Mutation(s): 0 | 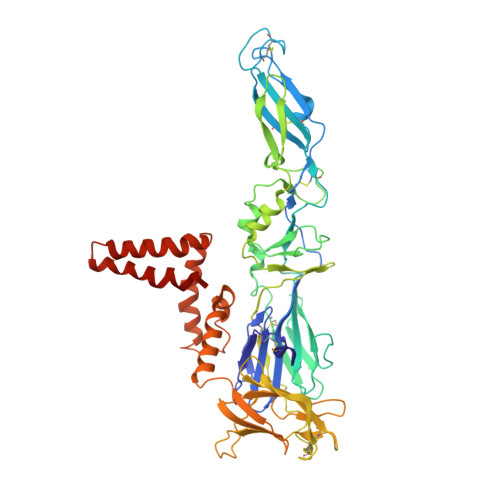 | |
UniProt | |||||
Find proteins for Q01299 (Tick-borne encephalitis virus (strain Hypr)) Explore Q01299 Go to UniProtKB: Q01299 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q01299 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by:
(by identity cutoff) | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Small envelope protein M | 75 | Tick-borne encephalitis virus (strain HYPR) | Mutation(s): 0 | 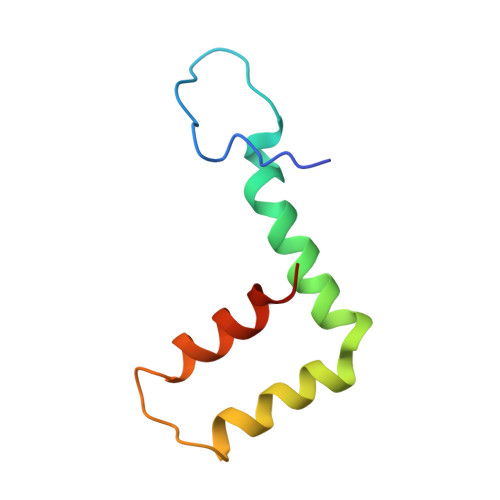 | |
UniProt | |||||
Find proteins for Q01299 (Tick-borne encephalitis virus (strain Hypr)) Explore Q01299 Go to UniProtKB: Q01299 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q01299 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by:
(by identity cutoff) | 3D Structure
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Fab 19/1786 - Heavy chain | G [auth H], H [auth I] | 212 | Mus musculus | Mutation(s): 0 | 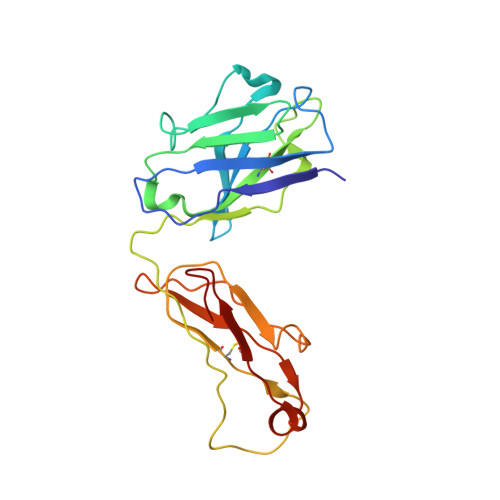 |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by:
(by identity cutoff) | 3D Structure
Entity ID: 4 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Fab 19/1786 - Light chain | I [auth L], J [auth M] | 208 | Mus musculus | Mutation(s): 0 | 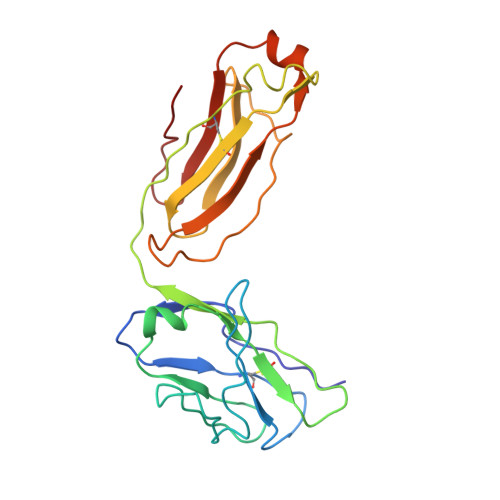 |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
Small Molecules
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| NAG Query on NAG | K [auth A], L [auth B], M [auth C] | 2-acetamido-2-deoxy-beta-D-glucopyranose C8 H15 N O6 OVRNDRQMDRJTHS-FMDGEEDCSA-N |  | ||
Experimental Data & Validation
Experimental Data
- Method: ELECTRON MICROSCOPY
- Resolution: 3.90 Å
- Aggregation State: PARTICLE
- Reconstruction Method: SINGLE PARTICLE
| Task | Software Package | Version |
|---|---|---|
| MODEL REFINEMENT | PHENIX | 1.11 |
| MODEL REFINEMENT | REFMAC | 5.8.0158 |
| RECONSTRUCTION | RELION | 1.4 |
Entry History & Funding Information
Deposition Data
- Released Date: 2018-02-07 Deposition Author(s): Fuzik, T., Plevka, P.
| Funding Organization | Location | Grant Number |
|---|---|---|
| European Research Council | 355855 | |
| European Molecular Biology Organization | Germany | IG3041 |
| Czech Science Foundation | Czech Republic | 17-02196S |
Revision History (Full details and data files)
- Version 1.0: 2018-02-07
Type: Initial release - Version 1.1: 2018-03-07
Changes: Database references, Structure summary - Version 1.2: 2018-10-17
Changes: Data collection, Refinement description, Structure summary - Version 1.3: 2019-11-06
Changes: Data collection, Derived calculations, Other - Version 1.4: 2019-12-11
Changes: Other - Version 1.5: 2020-07-29
Type: Remediation
Reason: Carbohydrate remediation
Changes: Data collection, Derived calculations, Structure summary













