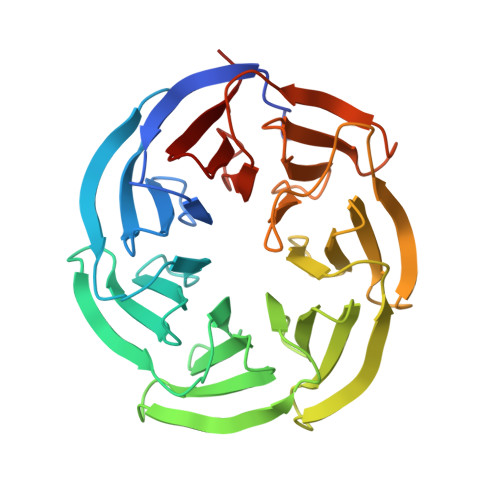
Structure of the WD40-domain of human ATG16L1.
Bajagic, M., Archna, A., Busing, P., Scrima, A.(2017) Protein Sci 26: 1828-1837
- PubMed: 28685931
- DOI: https://doi.org/10.1002/pro.3222
- Primary Citation of Related Structures:
5NUV - PubMed Abstract:
Autophagy-related protein ATG16L1 is a component of the mammalian ATG12∼ATG5/ATG16L1 complex, which acts as E3-ligase to catalyze lipidation of LC3 during autophagosome biogenesis. The N-terminal part of ATG16L1 comprises the ATG5-binding site and coiled-coil dimerization domain, both also present in yeast ATG16 and essential for bulk and starvation induced autophagy. While absent in yeast ATG16, mammalian ATG16L1 further contains a predicted C-terminal WD40-domain, which has been shown to be involved in mediating interaction with diverse factors in the context of alternative functions of autophagy, such as inflammatory control and xenophagy. In this work, we provide detailed information on the domain boundaries of the WD40-domain of human ATG16L1 and present its crystal structure at a resolution of 1.55 Å.
Organizational Affiliation:
Structural Biology of Autophagy Group, Department of Structure and Function of Proteins, Helmholtz Centre for Infection Research, Braunschweig, 38124, Germany.